Knee
Case 6.1 Articular Chondroma (Dysplasia Epiphysealis Hemimelica)
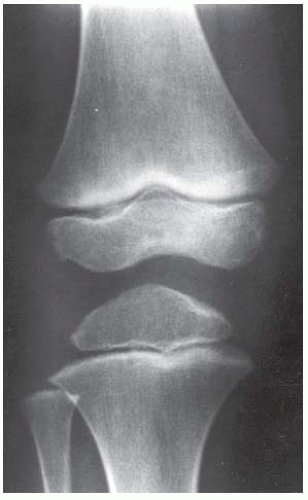
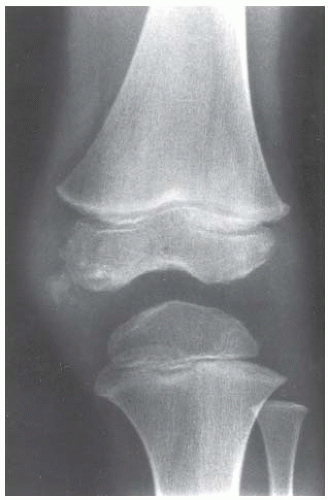
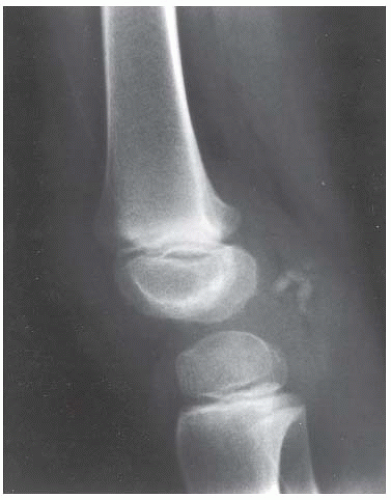
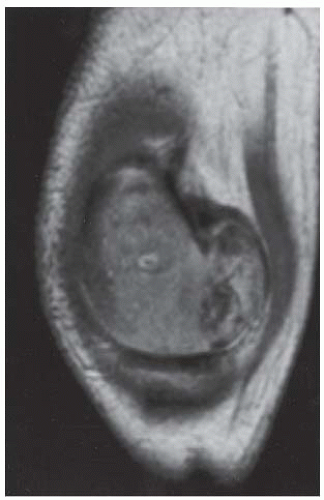
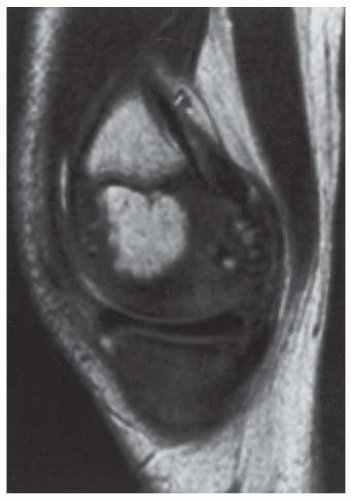
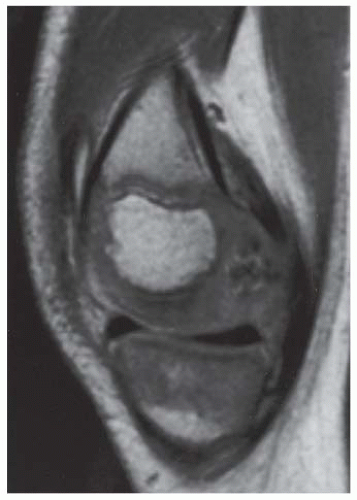
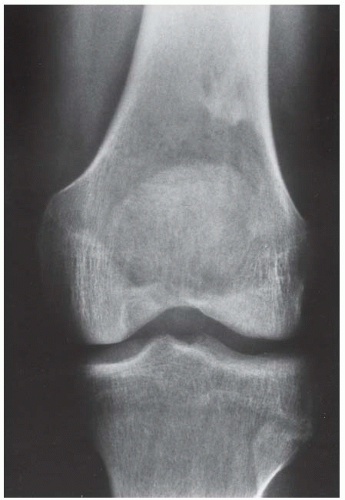
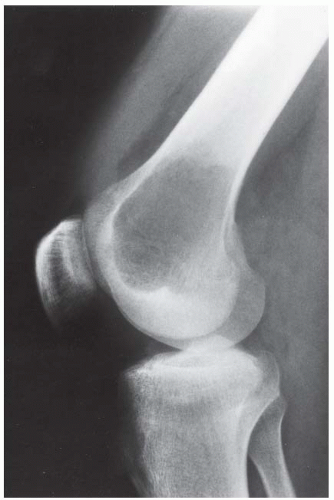
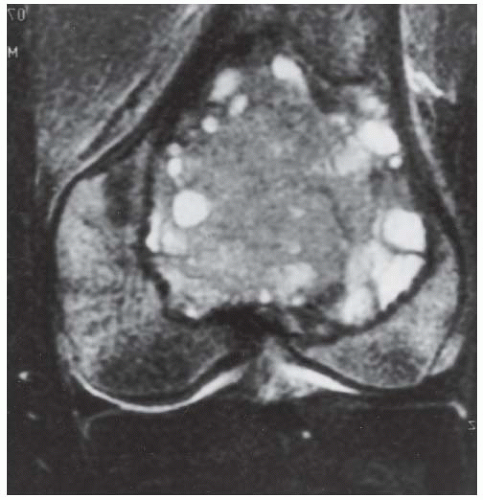
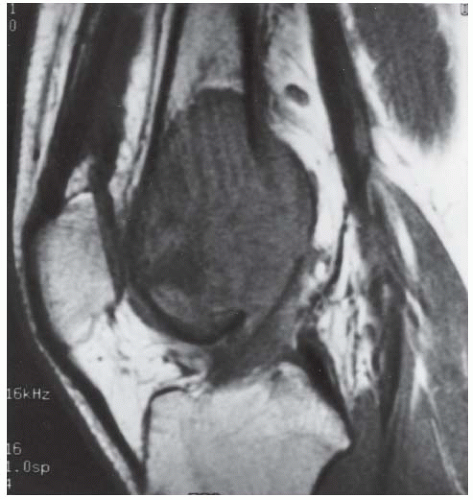
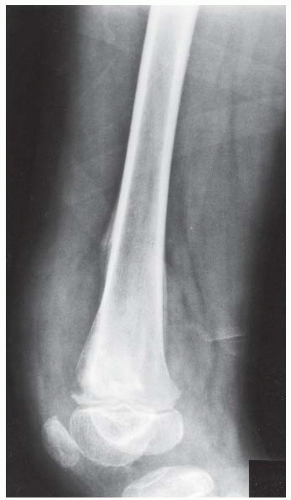
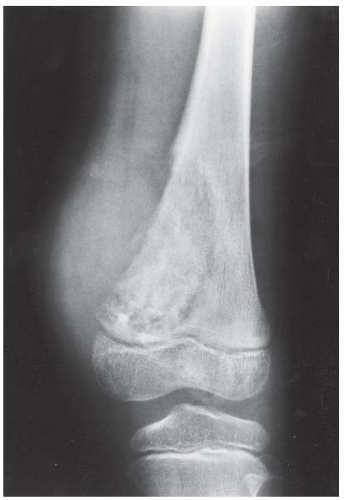
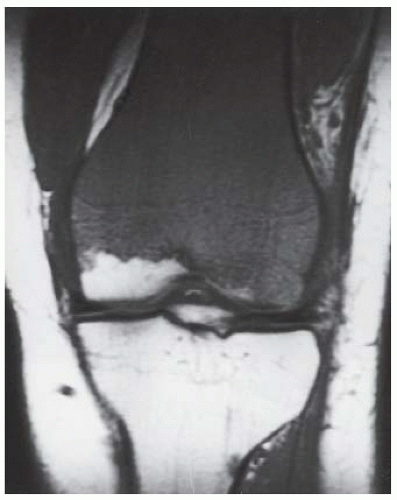
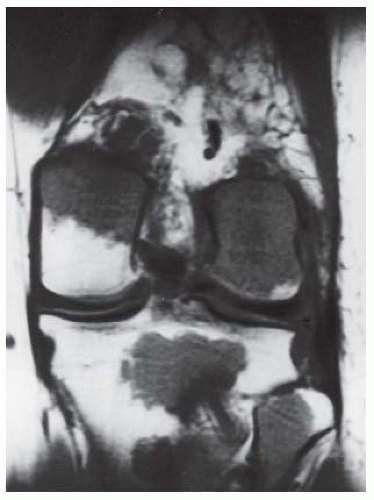
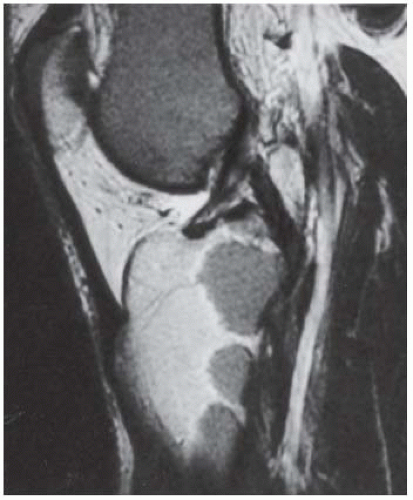
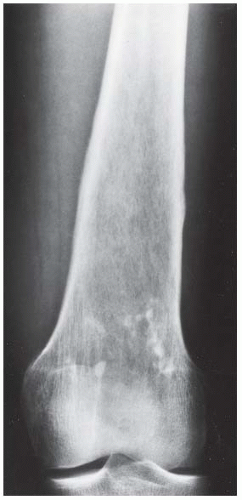
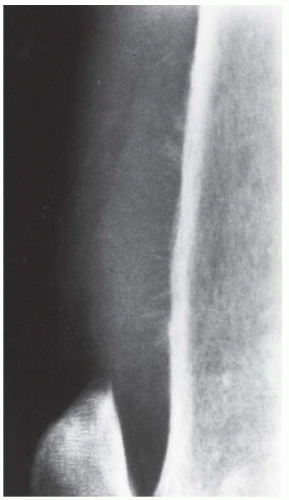
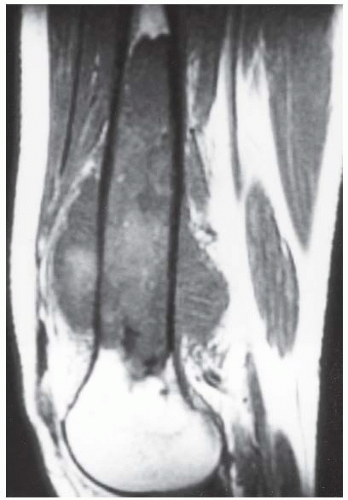
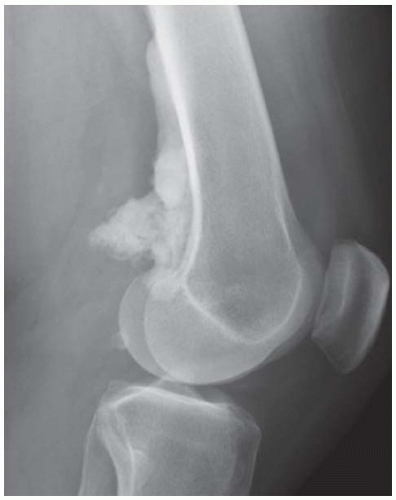
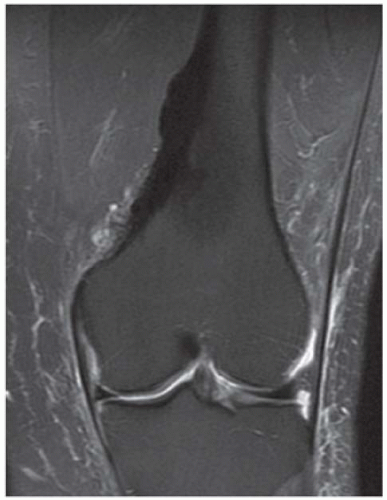
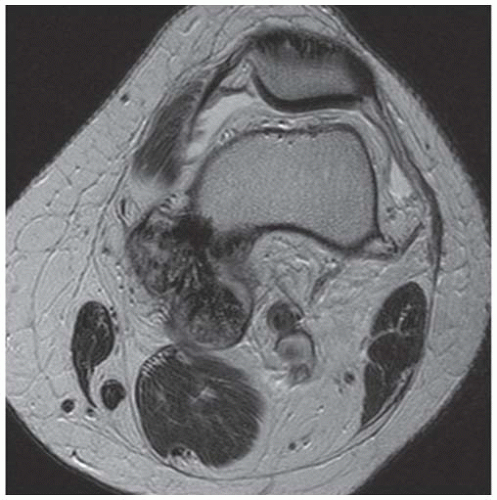
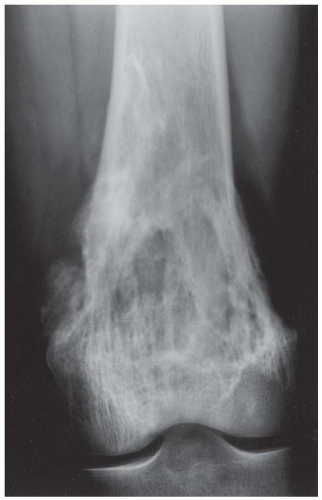
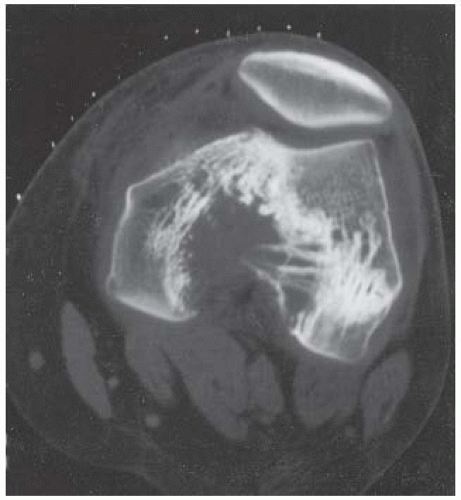
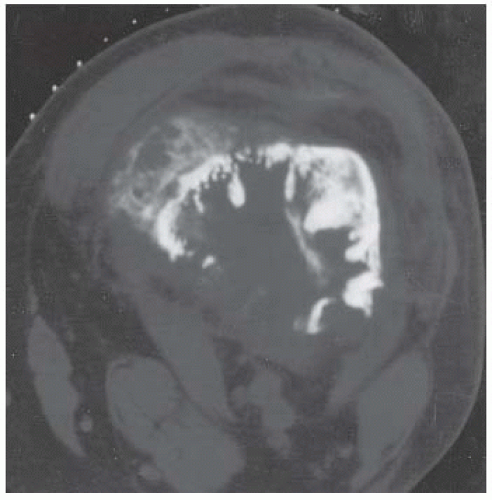
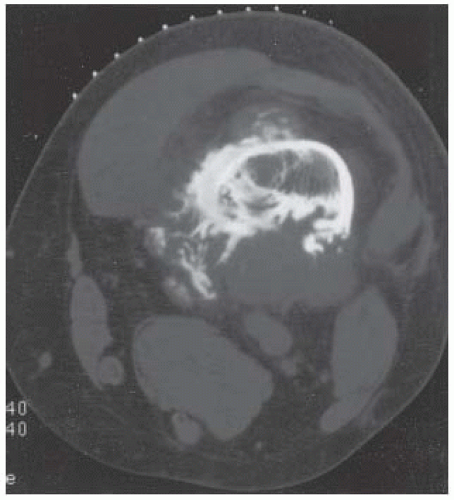
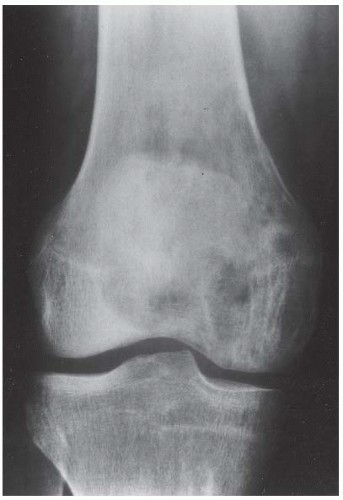
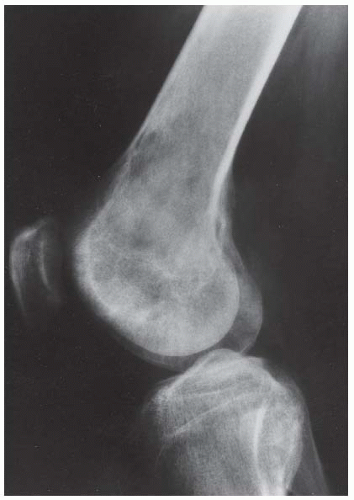
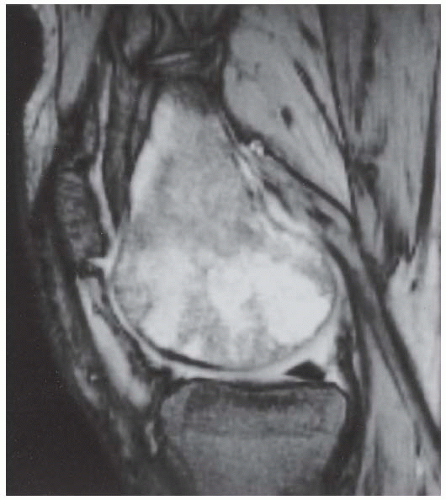
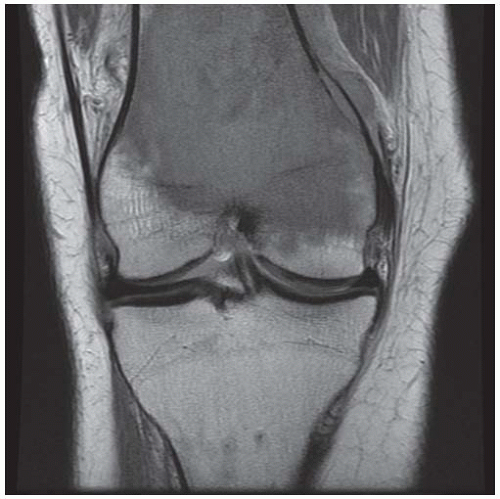
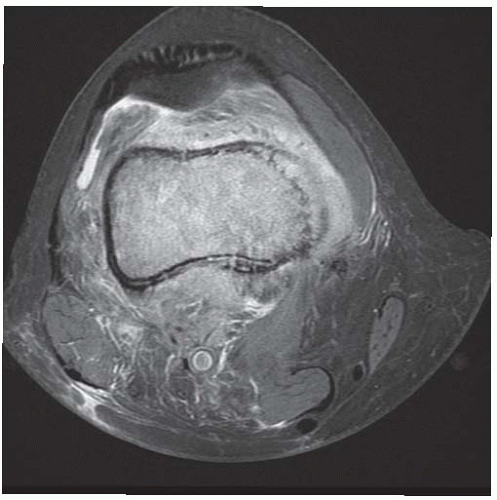
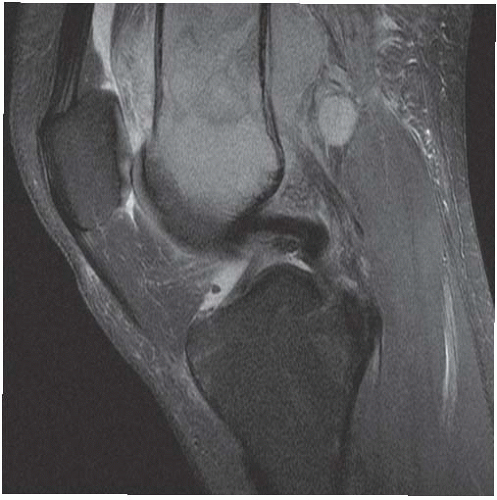
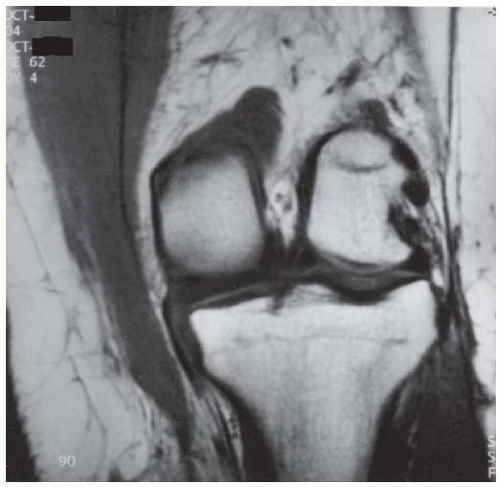
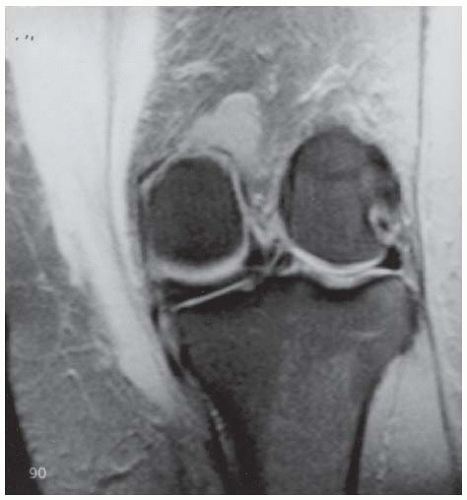
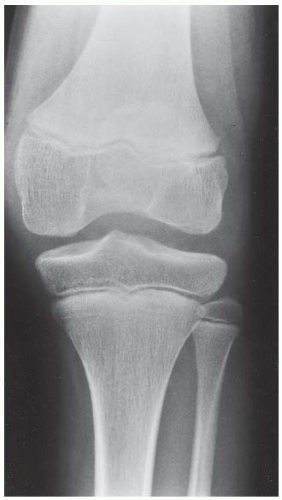
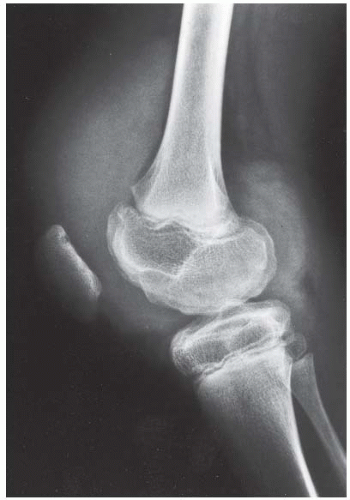
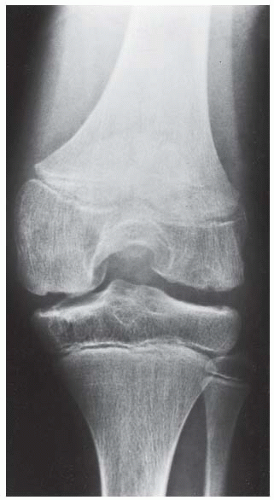
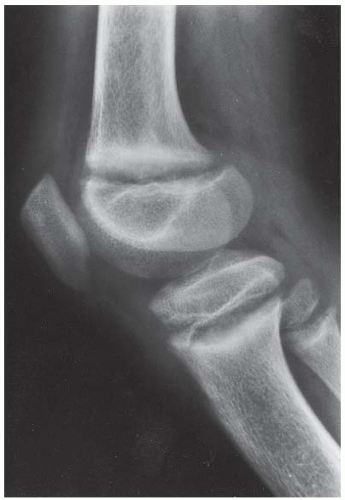
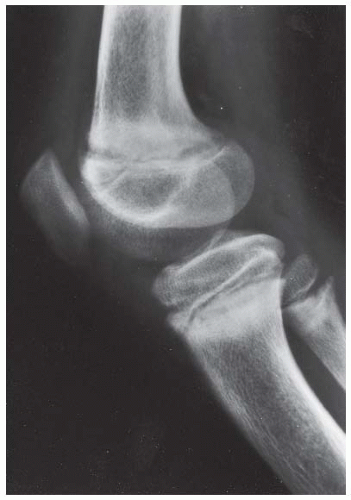
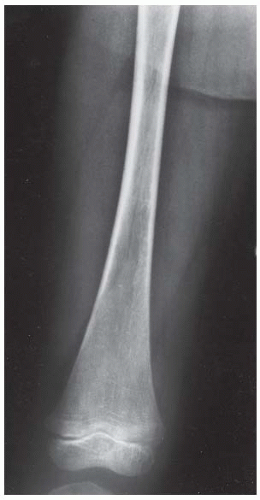
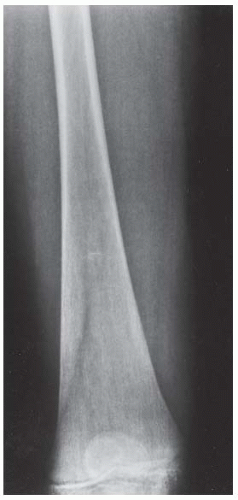
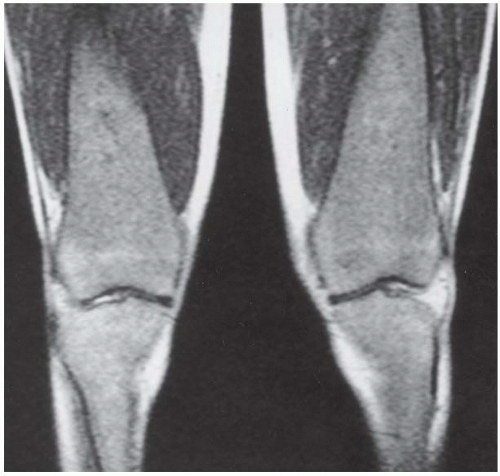
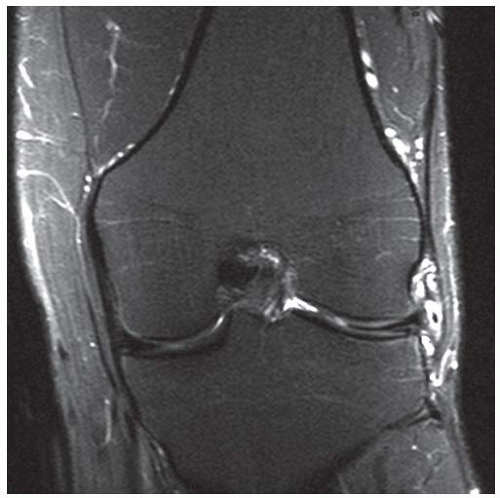
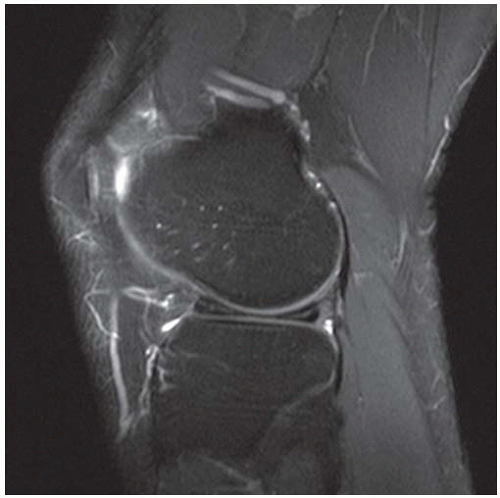
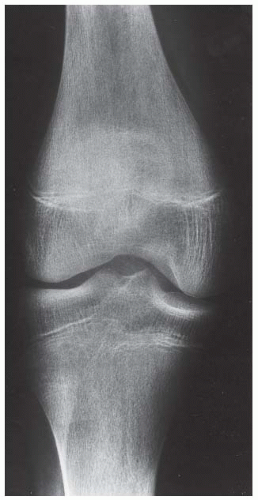
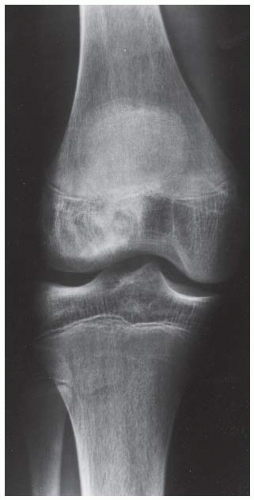
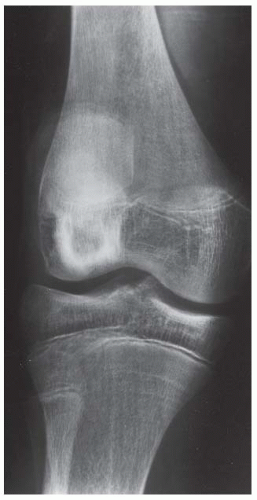
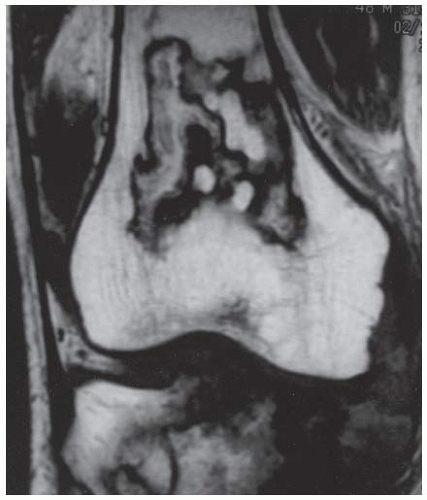
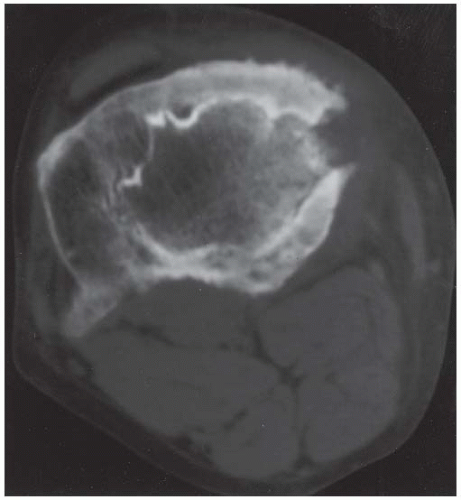
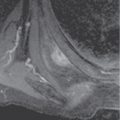
CLINICAL HISTORY A 3-year-old boy with swelling of the left knee. A, asymptomatic right knee radiograph; B-C, left knee radiograph; D-F, sagittal left knee MRI.
FINDINGS
A. Anteroposterior (AP) radiograph of the right knee is normal.
B, C. AP and lateral radiographs of the left knee demonstrate small, multifocal ossifications seen medial and posterior to the normal epiphysis.
D-F. Sagittal magnetic resonance imaging (MRI) through the medial femoral condyle using T1 weighting, proton density, and T1 weighting after intravenous gadolinium demonstrates cartilaginous overgrowth of the posterior aspect of the femoral epiphysis, with a secondary center of ossification. There is no mass lesion separate from this epiphyseal overgrowth.
DIFFERENTIAL DIAGNOSIS Articular chondroma, loose body, osteochondritis dissecans.
DIAGNOSIS Articular chondroma (dysplasia epiphysealis hemimelica).
DISCUSSION Articular chondroma, also called dysplasia epiphysealis hemimelica or Trevor disease [1], histologically consists of hyaline cartilage. It may be considered an epiphyseal form of osteochondroma. It is unilateral and typically affects only one-half of the epiphysis of a long bone (usually the medial portion), as seen here. The most common location is in the lower extremity, particularly about the ankle, which explains the alternate term of tarsal aclasis. It may or may not be associated with multiple exostoses. It is usually manifested in childhood. Complications include impaired growth and joint abnormalities, particularly deformities of alignment from the asymmetric growth. MRI is the most helpful imaging study in evaluating these lesions [2].
Case 6.2 Giant Cell Tumor
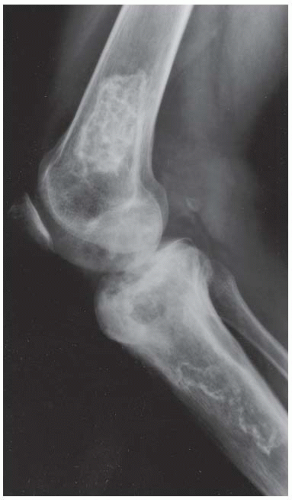
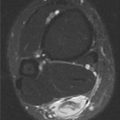
CLINICAL HISTORY A 22-year-old man with knee pain.
FINDINGS
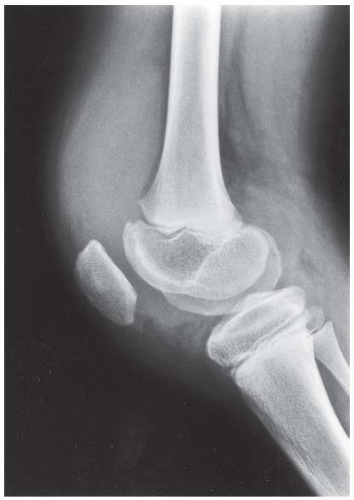
A, B. AP and lateral radiographs of the knee. There is a lucent lesion in the lateral femoral condyle. There is very little reactive bone, if any. The posterior cortex may be violated on the lateral view.
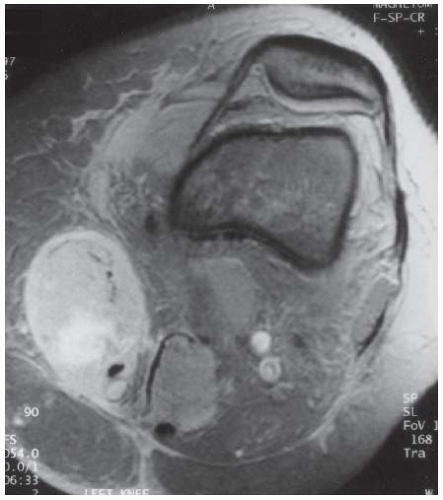
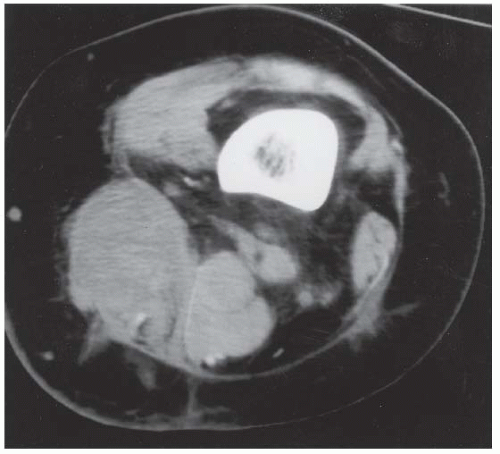
C, D. Coronal T2-weighted and sagittal T1-weighted MRI demonstrates T1 hypointensity and T2 isointensity to muscle, with multiple round areas of T2 hyperintensity at the margins.
DIFFERENTIAL DIAGNOSIS Giant cell tumor, chondroblastoma, clear cell chondrosarcoma.
DIAGNOSIS Giant cell tumor.
DISCUSSION The diagnostic possibilities for a large, solitary epiphyseal lesion in an adult include giant cell tumor, chondroblastoma, and clear cell chondrosarcoma. Chondroblastomas usually have sclerotic borders. Calcification is seen in 50% of patients, and periosteal reaction is seen in 50% of patients. Clear cell chondrosarcoma typically occurs in the proximal femur or proximal humerus. Therefore, the most likely diagnosis is giant cell tumor.
Giant cell tumor of bone (osteoclastoma) is an uncommon lesion thought to arise from osteoclasts. The presence of giant cells is only one histologic component of the tumor, and other types of tumors may have giant cells. Giant cell tumors can occur at any age, but the typical patient is a young adult. The location is almost invariably in the epiphysis, with extension to the subchondral cortex and into the metaphysis. Less than 2% of these tumors occur adjacent to open growth plates. Giant cell tumors probably arise in the cutback zone of the metaphysis, where osteoclasts are plentiful and active. About 50% of tumors are found about the knee, but other long bones and the sacrum are also commonly involved.
The typical radiographic appearance is a geographic, lytic tumor near the end of a long bone, extending to or very close to the subarticular cortex. Lytic regions correspond to nonmineralized tumor tissue, destroying and replacing cancellous bone. A lobular pattern of growth may leave ridges or trabeculations in surrounding bone. Giant cell tumors are often expansile and may have cystic blood-filled regions similar to aneurysmal bone cysts. The zone of transition from tumor to normal bone is usually sharp and abrupt, but without a sclerotic margin (growth rate I-B). Some lesions erode from the epiphysis into the joint cavity and provoke synovitis. Approximately 10% of patients present with pathologic fracture. Computed tomography (CT) or MRI may be required to show the extent of tumor and the relationship to the adjacent joint. Giant cell tumors appear as areas of intense isotope uptake on bone scan and sometimes have a doughnut appearance, with greater activity at the margins.
The typical treatment of giant cell tumor is curettage; adjuvant treatment of the surgical bed with a high-speed burr, phenol, or cryotherapy; and packing with methylmethacrylate. The reported overall rate of recurrence is about 25%. There are case reports of pulmonary and skin metastases from giant cell tumors [3,4]. Older literature suggests the existence of malignant giant cell tumors, but these may have represented primary malignant lesions such as osteosarcoma or malignant fibrous histiocytoma (MFH) that have prominent giant cells at histology. Spontaneous malignant transformation of a conventional giant cell tumor is rare [5].
Case 6.3 Osteosarcoma, High-Grade Intramedullary Type
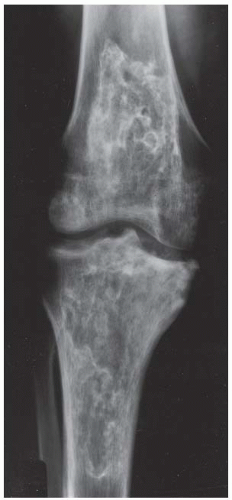
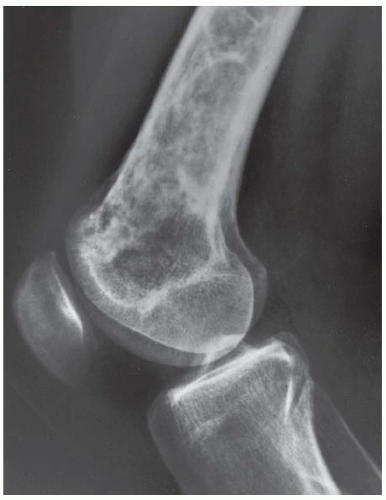
CLINICAL HISTORY A 6-year-old boy with pain and swelling at the knee for several months.
FINDINGS Lateral (A) and AP (B) radiographs of the knee. There is a large, destructive lesion involving the medial aspect of the distal femoral metaphysis, with cortical destruction and large soft tissue mass. The lesion extends to the growth plate but does not appear to cross into the epiphysis. Laminated, interrupted periosteal reaction is seen at the superior margin of the tumor. The margins of the lesion are poorly defined. Dense, amorphous regions of mineralization are present within the lesion.
DIFFERENTIAL DIAGNOSIS Osteosarcoma, Ewing sarcoma, lymphoma, metastasis.
DIAGNOSIS Osteosarcoma, high-grade intramedullary type.
DISCUSSION This destructive lesion should be unmistakable for a bone tumor. The lesion is moderately mineralized, and the mineralization has the dense, amorphous, cloud-like pattern characteristic of osteoid matrix. The location of the lesion and age of the patient is typical for the diagnosis. The age distribution of osteosarcoma has a sharp peak (46% of cases) between the ages of 10 and 20, but it has been described in very young children and in elderly adults. Osteosarcomas have been described in virtually every part of the skeleton, but the least common sites are probably the hands and feet.
In the Mayo Clinic series of bone tumors [6], the most common anatomic sites of osteosarcomas were distal femur (31%), proximal tibia (15%), proximal humerus (8%), pelvis (7%), proximal femur (5%), and femoral shaft (4%). Of osteosarcomas that occur in the long bones, only about 10% are found in the diaphysis alone, without extension to the metaphysis or epiphysis. The majority of osteosarcomas have no known cause, but in this series, more than 5% were found in irradiated bone, and more than 3% were found in regions of Paget disease. Patients older than 60 are much more likely to have a preexisting condition (38%).
Case 6.4 Polycythemia Vera
CLINICAL HISTORY A young adult woman with headache, dizziness, prolonged bleeding time, and hypertension.
FINDINGS (A, B) Coronal and (C) sagittal T1-weighted MRI demonstrate replacement of the normal fatty high-signal-intensity marrow with conglomerate deposits of low-signal-intensity material in both the femur and tibia. The visualized articular surfaces are normal.
DIFFERENTIAL DIAGNOSIS Polycythemia vera, multiple myeloma, lymphoma, leukemia, hemoglobinopathies.
DIAGNOSIS Polycythemia vera.
DISCUSSION Polycythemia vera is an idiopathic monoclonal marrow-proliferating process. Hyperplasia is most pronounced in the red blood cell constituents of the bone marrow. The main complications are thrombosis and bleeding diathesis. Multiple focal lytic lesions are characteristic. On MRI, replacement of the normal fatty marrow is reflected by decreased signal intensity on T1-weighted imaging, as seen here, showing an intermediate signal intensity on T2-weighted MRI between muscle and fat [7]. Later in the disease, marrow may be replaced by fibrosis and produce a clinical and radiologic appearance of myelofibrosis with extramedullary hematopoiesis. In this phase, there is decreased signal intensity in the bone marrow on both T1-weighted and T2-weighted MRI [8,9]. Secondary forms of polycythemia may occur and give rise to similar features. Gout, due to hyperuricemia, may complicate the radiographic findings. Definitive diagnosis is by bone marrow biopsy.
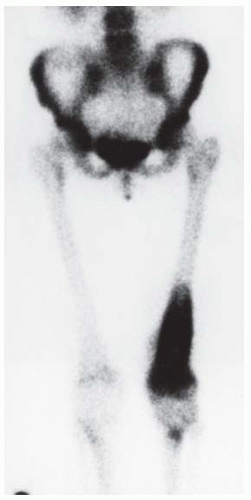
Case 6.5 Osteosarcoma, High-Grade Intramedullary Type
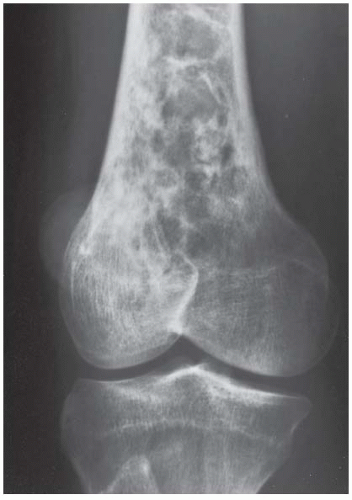
CLINICAL HISTORY A 20-year-old woman with knee pain and swelling for 3 months.
FINDINGS
AP radiograph of the distal femur. There is a mildly expansile lesion in the distal femur causing slight irregularity of the texture of the cortical bone, with a few localized areas of amorphous mineralization distally. There is subtle periosteal elevation medially and laterally at the distal shaft. The proximal and distal intramedullary extent of the lesion is imperceptible.
Lateral radiograph of the distal femur, photographic detail. There is permeation of tumor through the anterior femoral cortex into the soft tissues, with sunburst periosteal reaction and soft tissue mass. The destruction of the cortex is evident as unsharpness of its margins and vague lucency; the overall structure of the cortex remains intact.
Sagittal T1-weighted MRI shows an extensive intramedullary lesion with heterogeneous signal. The lesion penetrates the cortex anteriorly and posteriorly to form large soft tissue masses. Small regions of very low signal at the inferior extent of the tumor correspond to the mineralized matrix seen on the AP radiograph.
Radionuclide bone scan. Anterior whole-body scan (photographic detail) shows intense activity in the distal femur, corresponding to the tumor. There is also a modest regional increase in activity in the proximal femur and proximal tibia.
DIFFERENTIAL DIAGNOSIS Osteosarcoma, Ewing sarcoma, lymphoma, metastasis.
DIAGNOSIS Osteosarcoma, high-grade intramedullary type.
DISCUSSION This case illustrates a high-grade intramedullary osteosarcoma whose radiographic features are more subtle but nonetheless highly aggressive. The true extent of the lesion is shown dramatically by the MRI. The radionuclide bone scan shows a pattern of falsely extended uptake that corresponds to hyperemia and osteoporosis in the otherwise normal adjacent bone [10]. In contrast to the preceding cases, the lesion in this case is only slightly ossified.
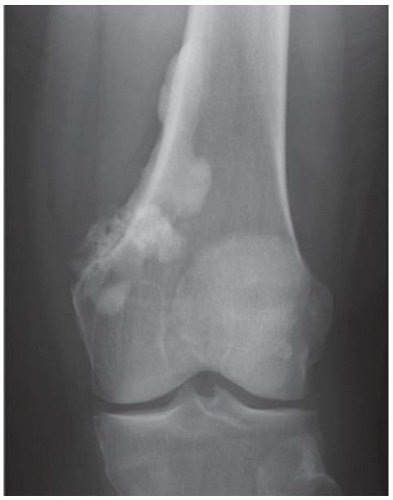
Case 6.6 Parosteal Osteosarcoma (Juxtacortical Osteosarcoma)
CLINICAL HISTORY A 24-year-old woman with mass behind her left knee.
FINDINGS
A, B. AP and lateral radiographs of the left knee show ill-defined dense osteoid densities centered about the posteromedial cortex of distal femoral metadiaphysis.
C, D. Coronalfat-saturated T2-weightedandaxial T2-weighted MR images show a T2-dark cortically based mass without intramedullary extension.
E. Axial CT (bone windows) shows a dense lesion arising from the posterior cortex of the left knee. The lesion has destroyed the cortex at the posterior medial margin of the femoral shaft, but there is no apparent tumor extending into the medullary space. The bulk of the tumor appears to be densely mineralized.
DIFFERENTIAL DIAGNOSIS Parosteal osteosarcoma, osteochondroma, periosteal osteosarcoma, high-grade surface osteosarcoma, myositis ossificans.
DIAGNOSIS Parosteal osteosarcoma (juxtacortical osteosarcoma).
DISCUSSION Parosteal osteosarcomas represent approximately 5% of osteosarcomas. They differ from the conventional high-grade intramedullary osteosarcoma in several significant ways. Parosteal osteosarcoma arises on the cortical surface rather than within the medullary space, and virtually all are found in the metaphysis of a long bone, especially the posterior surface of the distal femoral metaphysis (66% of cases). The peak age at diagnosis is in the third decade, with more than 80% of patients older than 20. The presentation is nonspecific— often dull aching pain or mechanical difficulties caused by the mass itself. The lesions are commonly diagnosed and treated incorrectly for years as atypical osteochondromas that somehow recur locally. Even with late diagnosis, the prognosis is often better than for conventional osteosarcoma because they are, by definition, of low histologic grade (a higher-grade osteosarcoma arising on the surface of bone would be classified by the pathologist as either a periosteal osteosarcoma or a high-grade surface osteosarcoma).
The radiographic appearance is a lobulated, juxtacortical mass with densely ossified tumor tissue attached to the cortex, often by a stalk. Variable amounts of lucent, nonossified tissue are usually present, making the lesion larger than apparent on plain radiographs. The peripheral portions of the lesion tend to have the least ossification. A cleavage plane between tumor and underlying bone may be visible at the edge of the stalk in approximately two-thirds of cases, and it is characteristic of the slow, lobular growth of the tumor as it becomes larger than its attachment to the bone. Tumor invasion of the medullary cavity may occur by direct extension through the stalk. Such invasion is found in the minority of cases and can often be documented by CT or MRI [11]. Localized regions of histopathologic dedifferentiation to high-grade spindle cell sarcoma is not uncommon (28%), either at presentation or at local recurrence [12]. The presence of a poorly defined soft tissue component distinct from the mineralized matrix is suggestive of a high-grade focus [13]. Parosteal osteosarcoma is the only malignant primary bone tumor that is more common in females than males (by a ratio of nearly 2:1 in the Mayo Clinic series) [12]. The treatment of parosteal osteosarcoma is surgical. Recurrences with simple curettage are common, but wide excision with clear surgical margins may be curative. Long-term survival rates of 80% to 90% may be expected for patients without regions of dedifferentiation.
Case 6.7 Osteosarcoma, Low-Grade Intramedullary Type
CLINICAL HISTORY A 35-year-old man with chronic, aching knee pain.
FINDINGS
AP radiograph of the knee. Bone loss is present at the intercondylar portion of the distal femur, probably extending to the medial cortex, with prominent reinforcement of the remaining trabeculae. Amorphous sclerosis is seen at the medial edge of the femur and along the more superior portion of the cortex adjacent to the intercondylar bone defect.
Axial noncontrast CT at the level of the femoral condyles shows a central destructive lesion with surrounding, sclerotic, reinforced trabecular bone. At the anteromedial cortex is a small, faintly mineralized soft tissue mass.
CT at the distal shaft shows extensive cortical bone destruction with prominent reinforced trabecular bone. Dense amorphous mineralization is seen on the endosteal surface of the anterior cortex. A mineralized soft tissue mass is present.
CT more proximally along the femoral shaft shows a large, partially mineralized soft tissue mass.
DIFFERENTIAL DIAGNOSIS Osteosarcoma, chondrosarcoma, lymphoma, metastasis, desmoplastic fibroma.
DIAGNOSIS Osteosarcoma, low-grade intramedullary type.
DISCUSSION This case has features of an aggressive lesion as well as an indolent lesion. The presence of cortical destruction, cortical penetration, and formation of soft tissue mass suggests an aggressive process. The presence of reinforced trabeculae compensating structurally for the regions of cortical destruction suggests a lesion that has been present for some time. The mineralization of the soft tissue portion of the lesion suggests a bone-forming tumor.
Low-grade osteosarcomas are well-differentiated bone-forming malignancies whose radiologic and histopathologic appearances are challenging to the diagnostician [12,14]. Rare lesions, they occur in adults and are typically found around the knee. The prognosis for long-term survival is excellent, but local recurrences may be accompanied by dedifferentiation to high-grade tumor and metastatic spread.
Case 6.8 MFH of Bone
CLINICAL HISTORY A 47-year-old man with knee pain.
FINDINGS
A, B. AP and lateral radiographs show a lytic lesion in the medial femoral condyle with ill-defined borders, but extending to the articular surface. An associated soft tissue mass is not noted, and there is no associated periostitis.
C. Sagittal T2-weighted MRI demonstrates high signal intensity in this lesion, without definite cortical violation.
DIFFERENTIAL DIAGNOSIS Malignant fibrous histiocytoma (MFH) of bone, metastasis, osteosarcoma, fibrosarcoma, chondrosarcoma, lymphoma.
DIAGNOSIS MFH of bone.
DISCUSSION MFH is a lesion that may occur in soft tissue or bone. MFH is considered to be of histiocytic origin. Histologically, the lesions show fibrogenic differentiation, and multinucleated malignant giant cells may be a prominent feature. MFH arising in bone is relatively rare, comprising only 1% of primary malignant bone tumors [12]. MFH more frequently arises in the soft tissues. The age range of patients is wide, but most patients are adults. Although most lesions occur around the knee, lesions have been reported in the extremities, spine, and skull. In long bones, the lesions are usually metaphyseal. As with other types of bone tumors, the typical clinical presentation is pain and swelling. Approximately 25% of MFH in bone is secondary to a preexisting pathologic process, most commonly previous radiation therapy, Paget disease, or bone infarction. Joint implants or their alloys have also been named as an inciting factor for MFH of bone [15].
The radiographic appearance is usually one of aggressive bone destruction with a moth-eaten or permeative pattern, as seen here. Reactive bone tends to be scant, and there will be no mineralized tumor matrix. The differential diagnosis includes metastasis, osteosarcoma, fibrosarcoma, chondrosarcoma, and lymphoma. The pathologic distinction between MFH and other bone sarcomas may be difficult. The presence of even microscopic foci of neoplastic osteoid or chondroid would cause the lesion to be classified as an osteosarcoma or chondrosarcoma, respectively. The distinction between MFH and fibrosarcoma is considered by some to be arbitrary, and they are radiologically indistinguishable.
Case 6.9 Primary Lymphoma of Bone
CLINICAL HISTORY A 52-year-old woman with knee pain for several weeks, without a precipitating episode of trauma.
FINDINGS
AP radiograph of the knee shows very subtle permeated destruction along the medial cortex of the femoral metaphysis.
Coronal T1-weighted MRI shows a large lesion replacing the normal fatty marrow, extending from the femoral shaft into the medial condyle. The periosteum is lifted by the lesion, and there is soft tissue extension.
Axial T2-weighted MRI with fat suppression shows high signal in the lesion, medial and posterior cortical destruction, soft tissue extension, and surrounding edema.
Sagittal proton density MRI with fat suppression shows anterior and posterior soft tissue involvement and an enlarged popliteal lymph node.
DIFFERENTIAL DIAGNOSIS Lymphoma, metastases, plasmacytoma, osteosarcoma, infection.
DIAGNOSIS Primary lymphoma of bone.
DISCUSSION Primary lymphoma of bone can have a near-normal appearance on radiographs [16]. MRI, as seen here, can show a large abnormality. In this case, the diagnosis was made by CT-guided needle biopsy. Sclerotic lymphoma of bone is one circumstance in which the percutaneously guided needle biopsy can be falsely negative. The infiltrative nature of the disease and the fragility of the cells may result in such severe crush artifacts that evidence of malignancy may be destroyed during the process of obtaining the specimen.
Patients with lymphoma involving bone may present with symptoms related to a bone lesion, or bone lesions may be discovered during staging after the diagnosis has been made from an extraskeletal site. Patients presenting with lymphoma of bone are considered to have primary lymphoma of bone if they have no evidence of the disease elsewhere. Primary lymphoma of bone constituted 3.9% of the cases of malignant bone lesions in the Mayo Clinic series [17]. There is a broad age range at time of diagnosis, and most lymphomas involve the portion of the skeleton containing red marrow. Bone destruction is the primary radiographic feature of primary lymphoma, and generally results in a permeated destruction of cortical and trabecular bone. A mottled and patchy appearance is typical. Approximately 50% of cases may have evidence of reactive bone or thickening of the cortex, but this reactive bone is typically sparse, and periosteal bone formation as might be seen in Ewing’s sarcoma is notably unusual. Soft tissue extension may be obvious, large, and asymmetric. Pathologic fracture is common. In a minority of cases, irregular sclerosis is present at the affected site, rather than a mixture of lysis and sclerosis.
Case 6.10 Non-Hodgkin Lymphoma
CLINICAL HISTORY A female college varsity soccer player with persistent pain after injury. The team doctor ordered an MRI.
FINDINGS
Coronal T1-weighted MRI shows enlargement of the sartorius muscle. The margins of the muscle are slightly irregular, and subcutaneous edema is present.
Coronal T2-weighted MRI shows high signal intensity in the sartorius muscle.
Axial T2-weighted MRI shows the enlargement of the sartorius, with high signal intensity and surrounding edema.
Axial CT at the time of needle biopsy shows enlargement of the sartorius.
DIFFERENTIAL DIAGNOSIS Non-Hodgkin lymphoma, intramuscular metastasis, soft tissue sarcoma, inflammatory disease, intramuscular tear and/or hemorrhage.
DIAGNOSIS Non-Hodgkin lymphoma.
DISCUSSION Factors that favor malignancy in a soft tissue mass are large size, deep location (e.g., intramuscular), and surrounding edema. The abnormality of the sartorius in this case has an aggressive appearance because of the diffuse nature of the enlargement and the markedly irregular margins around the muscle belly. The differential diagnosis includes inflammatory disease, intramuscular tear and/or hemorrhage, intramuscular metastasis, and soft tissue sarcoma. Lymphoma, presenting as an intramuscular mass without evidence of disease elsewhere, is a distinctly rare entity. Primary intramuscular non-Hodgkin lymphoma has a good prognosis, and because the treatment is not surgical, percutaneous needle biopsy is the key diagnostic procedure in this circumstance [18].
Case 6.11 Hemophiliac Knee
CLINICAL HISTORY A 9-year-old boy with swollen and painful knee. A-B, radiographs at presentation; C-D, radiographs one year later.
FINDINGS
A. Lateral radiograph of the knee shows marked synovial hypertrophy and effusion, with epiphyseal overgrowth.
B. AP radiograph shows squaring of the condyles and degenerative changes.
C, D. One year later. Lateral and AP radiographs demonstrate the progression of the findings, with the additional feature of erosions of the articular surface.
DIFFERENTIAL DIAGNOSIS Hemophilia, juvenile idiopathic arthritis, postinfectious arthropathy.
DIAGNOSIS Hemophilic knee.
DISCUSSION The early findings in this case may be attributable to juvenile idiopathic arthritis or hemophilia. Juvenile idiopathic arthritis is more common in females and generally shows osteoporosis as an early finding. Erosive changes are frequent in juvenile idiopathic arthritis and are not noted in the initial film, although joint destruction is already evident.
Hemophilia A is a clotting disorder related to factor VIII deficiency. It is transmitted as an X-linked disorder and therefore occurs almost exclusively in males. Although other varieties of hemophilia exist, this is the most common type. The primary radiographic feature of this disorder is hemarthrosis, as seen on the lateral views in this instance, with joint destruction after repeated episodes [19]. Some cardinal features include squaring of the inferior pole of the patella and widening of the intercondylar notch. These findings, however, can also be observed in juvenile idiopathic arthritis [20]. Differentiating features include subperiosteal hemorrhage and intraosseous cystic lesions that are not subarticular. The latter finding is related to intraosseous hemorrhage and, when substantial in size, is referred to as a pseudotumor. These are seen most often in the ilium and calcaneus. Extremity overgrowth or undergrowth may occur, depending on whether hyperemia or early fusion of the growth plate is the predominant effect of the disorder. Avascular necrosis of the epiphysis can occur secondary to elevated intra-articular pressures from hemarthrosis [21]. Avascular necrosis may occur in juvenile idiopathic arthritis as a result of steroid treatment.
Case 6.12 Rickets
CLINICAL HISTORY A 7-year-old girl with joint swelling. A, radiograph at presentation; B, radiograph 7 months later.
FINDINGS
Lateral knee radiograph taken at presentation. There is widening and fraying of the distal femur, proximal fibula, and tibia physes.
Lateral knee radiograph taken 7 months later. There is interval sclerosis and filling in of the physeal widening.
DIFFERENTIAL DIAGNOSIS Rickets, metaphyseal dysplasia.
DIAGNOSIS Rickets.
DISCUSSION Rickets and osteomalacia are childhood and adult manifestations, respectively, of a systemic disease in which the calcification of osteoid is deficient. The common final pathway in both conditions is the lack of available calcium or phosphorus (or both) for mineralization of osteoid. In rickets, the predominant effect is on the growth plates; in osteomalacia, the predominant effect is on remodeling of mature bone. When rickets or osteomalacia occurs in conjunction with chronic renal failure, the condition is called renal osteodystrophy.
Dietary deficiency of vitamin D, usually coupled with inadequate exposure to sunlight so that photochemical synthesis of vitamin D in the skin does not occur, results in reduced gastrointestinal calcium absorption, hypocalcemia, and secondary hyperparathyroidism to mobilize calcium from the skeleton. Pure vitamin D deficiency-induced rickets and osteomalacia is relatively rare in the United States, except among immigrants, food faddists, the institutionalized elderly, and patients on total parenteral nutrition. Other causes include failure of enzymatic conversion of 25-hydroxyvitamin D to its physiologically more active metabolite 1,25-dihydroxyvitamin D, end-organ insensitivity to 1,25-dihydroxy vitamin D, genetic and acquired renal tubular reabsorption defects, and gastrointestinal malabsorption of dietary calcium or phosphorus. In the United States, gastrointestinal malabsorption from a variety of etiologies is the most common cause of rickets and osteomalacia. Rickets and osteomalacia may occur in association with polyostotic fibrous dysplasia and neurofibromatosis, and may be caused by chronic use of anticonvulsant medications or aluminumcontaining antacids.
In rickets, there is widening of the growth plate because of continued cartilage growth in the absence of normal mineralization and ossification. Radiographic findings are most apparent in the most active regions of growth, and the uncalcified cartilage may become quite bulky. Frequent sites of radiographic abnormalities include the costochondral junctions of ribs, the distal femur, both ends of the tibia, the proximal humerus, the distal radius, and the ulna. Irregular, disorganized mineralization of the zone of provisional calcification creates a frayed appearance. Mechanical stress on the thickened growth plate may lead to widening, cupping, and bowing deformities. Bone texture (trabecular pattern) appears coarsened, and there is a delayed appearance of ossification centers. Rachitic bone is less resistant to bending and shearing loads, and stress fractures and bowing deformities are common. Transverse zones of lucency on the concave side of long bones, called Milkman pseudofractures or Looser zones, are focal collections of nonmineralized osteoid; they probably do not represent insufficiency injuries. After the initiation of successful treatment of rickets, the uncalcified osteoid calcifies, so that the zone of provisional calcification appears as a wide band that narrows the growth plate to its normal thickness. Ossification of nonmineralized subperiosteal osteoid is apparent as new periosteal bone.
Metaphyseal dysplasia is a rare disorder caused by an inborn error in enchondral ossification that results in widened, irregular-appearing growth plates. Laboratory values are normal, however.
Case 6.13 Gaucher Disease
CLINICAL HISTORY A 3-year-old boy with anemia and a companion case. A, radiograph at age 3; B, radiograph at age 12; C, companion case.
FINDINGS
AP radiograph of a knee taken at age 3 shows Erlenmeyer flask deformities of the femur and tibia.
AP radiograph of the knee taken at age 12 shows a progressive Erlenmeyer flask deformity.
Companion case. Coronal T1-weighted MRI of a young adult shows diffuse replacement of the fatty marrow, with intermediate signal and Erlenmeyer flask deformities.
DIFFERENTIAL DIAGNOSIS Gaucher disease, chronic anemias, Pyle disease, Niemann-Pick disease.
DIAGNOSIS Gaucher disease.
DISCUSSION Anemias, Pyle disease, Niemann-Pick disease, and Gaucher disease can produce Erlenmeyer flask deformities. The bone marrow is one of the largest organs of the body. Confined to the intramedullary space of bone, it consists of a meshwork of trabecular bone with fat cells, myeloid cells, reticulum cells, and supporting structures. At birth, the marrow cavities of the tubular bones, the flat bones, and the vertebrae have a predominance of hematopoietic cells. With advancing age, the hematopoietic marrow regresses and is replaced by fatty marrow, beginning distally in the extremities and progressing to incompletely encompass the pelvis, spine, and cranium. The process may reverse (called marrow reconversion) when there is an increased demand for hematopoiesis, as might occur in anemia or replacement of normal hematopoietic marrow by a pathologic process. Radiographic findings of marrow disorders are indirect and nonspecific.
When chronic marrow space expansion occurs in the growing skeleton, adaptive bone changes may occur during the development of the bone. Actual enlargement of the marrow space will alter the normal bony contours; such changes do not occur acutely, nor do they occur in the adult. MRI is the best method for direct imaging of the bone marrow. Because marrow is a conglomeration of different tissues, the appearance on MRI may vary, both with the composition of the marrow and the particular technical parameters. In general, fatty marrow has the predominant signal characteristics of fat, and hematopoietic marrow has signal characteristics more similar to muscle. Nuclear scans with technetium Tc-99m sulfur colloid or technetium Tc 99mTc methylene diphosphonate can provide physiologic assessments of the reticuloendothelial marrow elements and the surrounding bone, respectively.
The prototype for the lipid storage diseases is Gaucher disease (glucocerebroside lipidosis). In this autosomal recessive condition, deficiency of glucocerebrosidase results in the progressive accumulation of histiocytes laden with glucocerebroside lipids in the bone marrow and other organs and tissues. Secondary changes in bone are observed [22]. The classic radiographic finding is the Erlenmeyer flask deformity, which is undermodeling of the metaphysis due to marrow space packing. Cortical thinning by endosteal erosion and osteopenia are additional radiographic abnormalities that may be evident. Osteonecrosis of the femoral head is a common association, and is usually bilateral. After prolonged enzyme replacement therapy with macrophage-targeted glucocerebrosidase (glucosylceramidase), marrow composition, bone mass, and bone morphology revert toward normal.
Case 6.14 Discoid Meniscus with Complex Tear
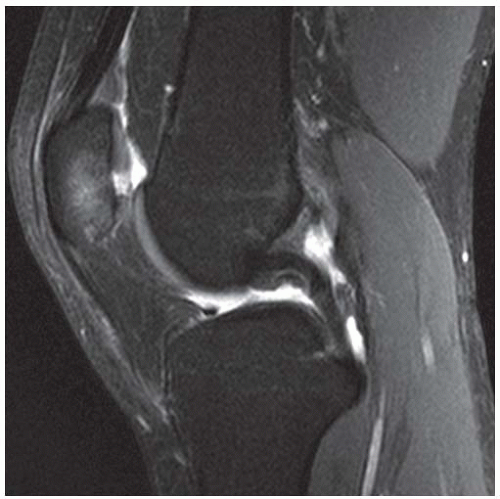
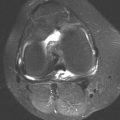
CLINICAL HISTORY A 38-year-old man with lateral knee pain.
FINDINGS (A, B) Coronal inversion recovery, and sagittal proton density, fat-suppressed MRI demonstrates a large lateral meniscus with diffusely abnormal signal.
DIFFERENTIAL DIAGNOSIS Discoid meniscus, complex meniscal tear.
DIAGNOSIS Discoid meniscus with complex tear.
DISCUSSION A discoid meniscus is a developmental anomaly that predisposes the patient to meniscal tears. Instead of the meniscus being C shaped, it is shaped like a disc. With meniscal tissue now present in the joint space, it is more likely to degenerate and tear. The majority of adolescent patients with a meniscal tear have this anomaly. A discoid meniscus appears abnormally large on MRI. It will measure at least 12 mm wide on coronal images and have three or more sagittal images (4 mm thickness each) with a bow-tie appearance. The discoid meniscus is typically lateral in location and does not predispose the patient to tears of the nondiscoid medial meniscus [23]. Treatment of symptomatic patients includes repair of a tear, if present, and saucerization of the excess meniscal tissue [24].
Case 6.15 Sickle Cell Anemia
CLINICAL HISTORY An 8-year-old boy with knee pain; radiographs are 5 months apart. A, radiograph at presentation; B, radiograph 5 months after presentation; C, radiograph 10 months after presentation.
FINDINGS
AP film of the right knee demonstrates normal articular surfaces, but medial femoral condyle overgrowth.
AP radiograph of the right knee taken 5 months later demonstrates increased density to the lateral femoral condyle, with a smooth subarticular band.
AP radiograph of the right knee taken 10 months later demonstrates a more defined, solid band of sclerosis within the femoral condyle.
DIFFERENTIAL DIAGNOSIS Bone infarct due to hemoglobinopathy, Gaucher disease, steroid medication, trauma, pancreatitis.
DIAGNOSIS Sickle cell anemia.
DISCUSSION Sickle cell anemia occurs when valine is substituted for glutamate in the beta chain of hemoglobin. The result is an abnormal form of hemoglobin that is less effective at carrying oxygen and predisposed to causing thromboses. The skeletal manifestations of this process include widening of the marrow space, bone infarcts, and an overall dense appearance of the bones. H-shaped vertebra and a snow-capped appearance of the humeral heads is classic. Osteomyelitis is not uncommon, and the diaphysis represents a common site for its occurrence in this condition. Salmonella is a more frequent pathogen in these cases. The main complication is infarctions due to the abnormal configuration of the hemoglobin, as seen here.
Case 6.16 Calcified Medullary Infarcts with Subchondral Collapse of the Tibial Articular Surface
CLINICAL HISTORY A 38-year-old woman treated with corticosteroids for inflammatory arthritis.
FINDINGS
A, B. Lateral and AP radiographs of knee. There are extensive regions of irregular calcification in the distal femur and proximal tibia. There is no mass effect or bone destruction.
C. Coronal T1-weighted MRI shows the lesions with low-signal-intensity borders and fatty centers.
D. Axial CT through the tibial epiphysis shows a calcified central lesion with well-defined, serpentine, sclerotic borders and more irregular sclerosis in the surrounding bone.
DIFFERENTIAL DIAGNOSIS None.
DIAGNOSIS Calcified medullary infarcts with subchondral collapse of the tibial articular surface.
DISCUSSION Ossification around the margins of medullary infarcts occurs after revascularization and repair. Bone repair occurs as a process of creeping substitution, and the sclerotic margin represents the portion being repaired. New bone is layered on the infarcted trabeculae, which are then removed very slowly and replaced by living bone. This process is generally too slow to observe progression on serial radiographs. Complications related to bone infarction include subchondral collapse, secondary osteoarthrosis and related sequelae, and the rare development of sarcomas such as osteosarcoma or MFH [25].
Case 6.17 Radiation Changes
CLINICAL HISTORY A 30-year-old woman with history of lymphoma.
FINDINGS A, B. Lateral (A) and AP (B) radiographs of the knee. There are sclerotic and cystic-appearing changes in distal femoral metaphysis. The trabecular pattern appears coarsened. There is no cortical destruction, periosteal reactive bone, or soft tissue involvement.
DIFFERENTIAL DIAGNOSIS Radiation changes, marrow infarcts, Paget disease, metastases, osteosarcoma, lymphoma, infection.
DIAGNOSIS Radiation changes.
DISCUSSION A mixed lytic and sclerotic pattern may be seen in Paget disease, metastases, certain primary bone tumors, infection, and radiation. There is no cortical thickening, however, making Paget disease less likely. There is no cortical destruction to suggest tumor or infection.
Therapeutic irradiation is a common treatment for osseous metastases. Sites of bone pain confirmed as abnormal by radiographs or bone scan in patients with known metastases are often radiated as palliative treatment. Irradiated osseous lesions heal by sclerosis and filling in of lytic areas. Radiation effects are independent of the source of the radiation. In the immature skeleton, radiation in total doses of 2,000 cGy or more impairs bone growth. The epiphysis is particularly sensitive, since radiation causes direct cellular injury to chondrocytes, and possibly vascular damage to fine physeal blood vessels. The greater the growth potential at the time of irradiation, the more profound the effect. If an entire growing bone is irradiated, loss of bone growth in the whole bone results in a small bone. Focal doses affect the irradiated portion; for example, angular deformities could result from an asymmetrically irradiated growth plate. Radiotherapy also increases the risk for epiphyseal plate trauma, including the occurrence of slipped capital femoral epiphysis and avascular necrosis. Scoliosis may follow irradiation of the spine.
In the mature skeleton, the primary complication is radiation osteonecrosis. This is a dose-related effect and occurs due to effects on osteoblasts. The mandible is a common site of involvement, and radiation osteonecrosis occurs more frequently after treatment for oral cancers as opposed to other head and neck tumors [26]. Treatment of radiation-induced osteonecrosis with hyperbaric oxygen may be of benefit [27]. Radiographs and CT will show irregular sclerosis in the irradiated bone. Radiation osteitis shows predominantly sclerosis and periostitis, and predisposes the patient to ischemic necrosis, infection, and fracture.
On bone scan, irradiated bone may initially show increased radionuclide accumulation from hyperemia and new bone formation. After several weeks or months, the bone scan will show decreased radionuclide accumulation due to decreased bone formation and decreased vascularity. On MRI, irradiated bone has the signal characteristics of fatty marrow. The anatomic location and extent of these changes conforms to the size and shape of the radiation portal.
Case 6.18 Chondromalacia Patellae
CLINICAL HISTORY A 33-year-old woman with acute-onset knee pain.
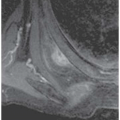
FINDINGS (A, B) Axial and sagittal proton density, fatsaturated MRI demonstrates a fissure and full-thickness loss of the articular cartilage along the patellar ridge associated with subchondral edema.
DIFFERENTIAL DIAGNOSIS None.
DIAGNOSIS Chondromalacia patellae.
DISCUSSION Chondromalacia patellae refers to patellar cartilage damage causing pain. Many different staging methods have been proposed. A method described by Outerbridge [28] is commonly used.
Stage 1: Softening or swelling of the cartilage, seen as signal intensity changes on MRI.
Stage 2: Fragmentation or fissuring of the cartilage measuring half an inch or less.
Stage 3: Fragmentation or fissuring of the cartilage measuring greater than half an inch.
Stage 4: Full-thickness cartilage loss.
Case 6.19 Rheumatoid Arthritis
CLINICAL HISTORY A 55-year-old woman with knee pain.
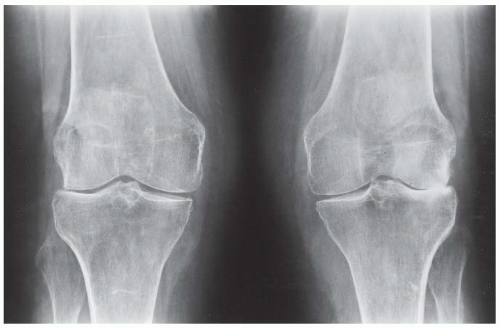
FINDINGS AP standing radiograph of both knees. The bones are osteoporotic. Uniform joint space loss is present with minimal proliferative bone changes. Some secondary osteoarthritic changes are present in the lateral compartment of the left knee, but these are rather modest compared to the amount of joint space loss.
DIFFERENTIAL DIAGNOSIS Rheumatoid arthritis, osteoarthritis, pyrophosphate arthropathy.
DIAGNOSIS Rheumatoid arthritis.
DISCUSSION This case shows features of systemic inflammatory arthritis, with uniform cartilage space loss and osteoporosis. The presence of subchondral sclerosis and modest osteophyte formation is indicative of secondary degenerative changes, a common feature in rheumatoid arthritis that involves the hips and knees.
Rheumatoid arthritis is a systemic autoimmune disease manifested in the musculoskeletal system by inflammatory polyarthritis of the small synovial joints. The pathogenesis is not understood and no causative agent has been proven. Genetic factors affect the susceptibility to, and expression of, the disease. Rheumatoid arthritis is generally distinguished from other arthritides by the presence of rheumatoid factor (RF) in the serum. Rheumatoid arthritis has a prevalence of 1% in the general population, with women affected more often than men by a ratio of 3:1. High RF titers often correlate with more severe disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree