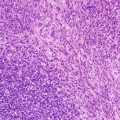
Fig. 1.1
CD99 immunohistochemistry in a case of Ewing sarcoma family tumor demonstrates diffuse strong expression of the CD99 antigen by tumor cells
Techniques to produce quality antibodies for clinical immunohistochemistry have improved dramatically over the past few decades. Antibodies against a specific antigen can be monoclonal or polyclonal, and they may be produced in a variety of hosts (commonly mouse or rabbit) against a wide array of epitopes. In comparison with the nascent years of IHC technology a few decades ago when frozen tissue was required and manual staining methods were predominant, immunostaining techniques are currently much more robust, automated, and amenable to being performed on a variety of tissue fixatives and tissue processing techniques. Nonetheless, IHC quality remains a function of a broad range of factors that include antibody specificity, antibody dilution and incubation conditions, antigen retrieval, tissue fixation, decalcification methods, and histologic processing [4]. For example, the length of tissue fixation and type of fixative might significantly alter a target epitope and thus impact IHC quality [5, 6]. Tissue processing techniques may similarly impact IHC particularly when novel techniques such as microwave are introduced.
More recently, colorimetric in situ hybridization (ISH) stains have become widely available. These stains are typically performed on the same automated platforms on which IHC is done. Instead of an antigen-antibody design, ISH entails the use of chromogen-tagged nucleic acids complementary to target DNA or RNA sequences [7]. Like IHC, ISH permits the identification of target sequences in a tissue-specific context. Commonly used ISH stains include those for the detection of human papillomavirus DNA, Epstein-Barr virus RNA, and immunoglobulin light-chain mRNA transcripts.
Interpretation of IHC requires a thorough knowledge of histology, antigen distribution in tissue, and antigen distribution in cells (membranous, cytoplasmic, and/or nuclear), and knowledge of potential artifacts that may impact staining quality. Accordingly, it is necessary to distinguish true staining from nonspecific cross-reactivity or background “noise.” Required elements to ensure adequate IHC quality include the use of positive and negative controls as well as systematic validation processes to ensure that critical components of IHC staining (e.g., buffers, color development kits) are performing optimally.
Flow Cytometry Analysis
Multicolor flow cytometry analysis (FCA) is an invaluable laboratory tool for the characterization of hematolymphoid malignancies. It permits multiparametric measurement of cellular properties that include size, cytoplasmic complexity, and antigen expression. A typical flow cytometer is composed of a laminar flow cell transport system, one to several laser lights, photodetectors, and a computer-based data management system. The intricate design of flow cytometers ensures that cells flowing in a fluid sheath are hydrodynamically focused to intercept laser light at a specific frequency. The interaction of the laser light with the cell results in light scatter and, in the presence of bound fluorochrome-tagged antibodies, excitation and resultant emission of light at a different wavelength. These events are captured by sensitive photodetectors and converted to measurable parameters. Scattered light captured by a detector positioned at a right angle (90°) from the laser source measures cytoplasmic complexity whereas scattered light captured by detectors along the original trajectory of the laser beam (180°) measures cell size.
Flow cytometry analysis is a robust tool to simultaneously assess coexpression of multiple antigens expressed by cells (Fig. 1.2). This is useful to many clinical assays including cell lineage determination, biomarker detection, minimal residual disease assessment, enumeration of cell subsets (e.g., stem cells, T-cell subsets), and measurement of proliferation and apoptosis.
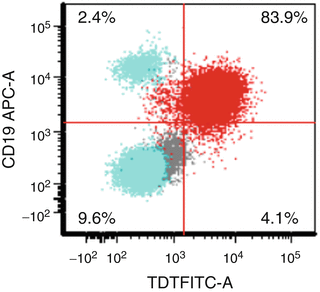

Fig. 1.2
Flow cytometry analysis of a case of B lymphoblastic leukemia/lymphoma demonstrating TdT expression by CD19-positive blasts (red). In this plot, the lymphocyte gate is highlighted in turquoise. Note the presence of a small population of normal CD19-positive B cells (upper left–hand quadrant) as well as a population of normal T cells (CD19 negative) (lower left-hand quadrant)
For such applications, antibodies with covalently linked fluorescent molecules (fluorochromes) are used to identify target antigens and provide a means for qualitative and quantitative assessment of antigen expression. This ability to perform multiparametric analysis on an individual cell offers FCA a distinct advantage over immunohistochemistry particularly in hematolymphoid disorders [8]. On the other hand, the use of FCA to evaluate solid tumors remains technically limited.
Cytogenetics
Conventional cytogenetic analysis (cytogenetics) is a laboratory discipline that involves the study of chromosomes, also known as karyotyping. Chromosomal alterations are common in cancer and are broadly categorized into recurrent and nonrecurrent abnormalities. Tumors arising in the pediatric age group are more likely than those arising in adults to harbor recurrent cytogenetic abnormalities. Frequently, such recurrent cytogenetic abnormalities are integral elements of pediatric cancer pathogenesis and their detection has emerged as an important adjunct for diagnostic evaluation.
For conventional cytogenetic analysis of tissue samples viable fresh cells are required for analysis. The average viable human cell divides once every 24 h and certain cell types, such as lymphocytes, do not divide at all, which mandates special culture techniques and growth stimulation of the cell of interest to increase the yield of analyzable material. Bone marrow is typically cultured for 24–48 h whereas lymphocytes from tissue may require 3–4 days in culture medium containing proliferation inducers for maximum yield. Cultured cells are then subjected to metaphase arrest before being processed to prepare chromosome spreads. Chromosomes are then stained, most commonly with Giemsa or Wright stains, for visualization of the characteristic banding patterns. Positively charged dyes in stains bind to the negatively charged DNA in chromosomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree