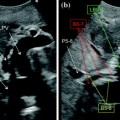
Fig. 12.1
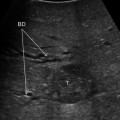
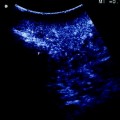
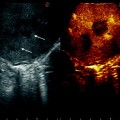
Patient with a 5 cm colorectal liver metastasis in Sg2–3. Preoperative CT (a) and MRI (b) showing the liver metastasis (T) and a 10 mm suspected lesion (arrow) in Sg7 close to a branch of the right hepatic vein draining Sg7 (V7). The suspected lesion shows CT and MRI features similar to the main lesion. c Laparoscopic ultrasound of the Sg2-3 metastasis (T). d The suspected lesion in Sg7 (arrow) is clearly identified as a haemangioma showing typical US features (uniform hyperechogenicity, well-defined margins and posterior sonic enhancement)
Then, additional lesions have to be excluded (Fig. 12.2). The exploration of the liver must be systematic and visualize all the segments. The authors suggest to perform LUS from left to right (from left lateral to the right posterior segments, including segment 1). Attention should be paid to the visualization of segment 1 and paracaval portion of segment 7 because of deep location. The identification of the posterior glissonean sheath guarantees a complete exploration. Additional neoplastic lesions usually have characteristics similar to those of known nodules. It should be kept in mind to adequately characterize images, especially in patients with nonhomogeneous liver parenchyma (cirrhotic patients or after prolonged chemotherapy) that may have many pseudonodular images.
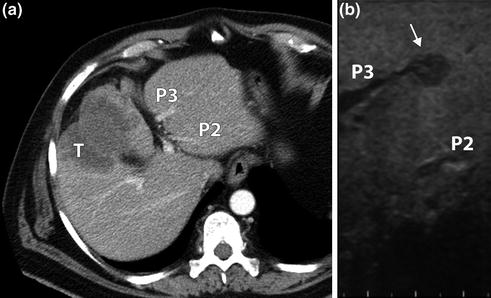

Fig. 12.2
CT scan (a) showing a gallbladder cancer (T) infiltrating Sg4b-5 without intrahepatic lesions. LUS (b) identified an intrahepatic metastasis (arrow) missed at CT infiltrating Sg3 portal branch (P3). P2: Sg2 portal branch
Once all the lesions have been detected, their relationships with vascular and biliary structures have to be analyzed. This aspect has been almost completely neglected in the literature. In some patients preoperative imaging cannot distinguish if a neoplastic lesion is just contiguous or infiltrates adjacent structures. It can be even more critical in new detected lesions, for which no data other than intraoperative ones are available. Thanks to magnificated images and multiplanar study, LUS may clarify these aspects. The degree of circumferential contact, the loss of hyperechoic peripheral boundary in vessels and an upstream dilatation of biliary ducts may be predictive of infiltration. Some tumors tend to grow into hepatic or portal veins (hepatocellular carcinoma) or biliary ducts (cholangiocarcinoma). LUS can disclose an unknown tumor thrombus (Fig. 12.3) or better characterize a suspected one (Fig. 12.4). The relationships with surrounding structures are even more important in perihilar lesions that grow just beside the main vessels and bile ducts. Not only the intrahepatic extension of the tumor has to be assessed but also it is spread along the hepatic pedicle. Every single anatomic structure has to be identified and studied. LUS is able to confirm an infiltration, reproducing the same images as preoperative ones (Fig. 12.5). The possibility to achieve a conclusive diagnosis in doubtful cases (contact vs. infiltration) can be inferred. Bile ducts can also be accurately evaluated. In hilar cholangiocarcinomas the extension along biliary tree is one of the main determinants of their resectability. LUS can clearly identify the borders of tumor, especially when bile ducts are dilatated (Fig. 12.6). In the authors’ opinion, LUS can accomplish a complete staging even for these complex diseases (Fig. 12.7). To date, no study confirmed these hypotheses and surgical exploration with direct dissection remains the standard. Prospective analyses are needed to confirm potentialities of LUS.
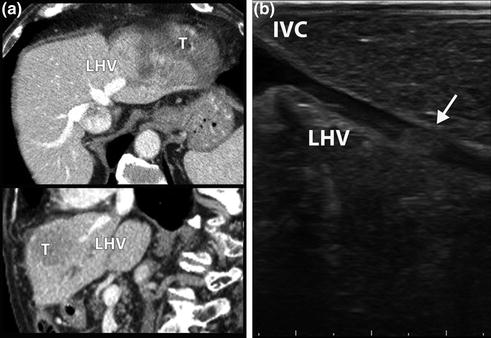
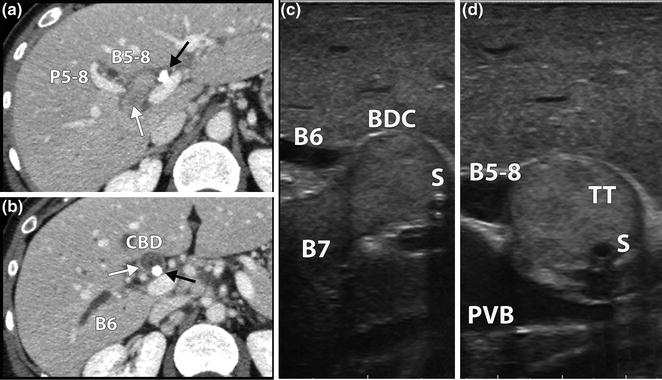
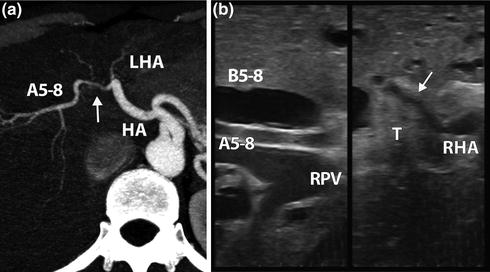
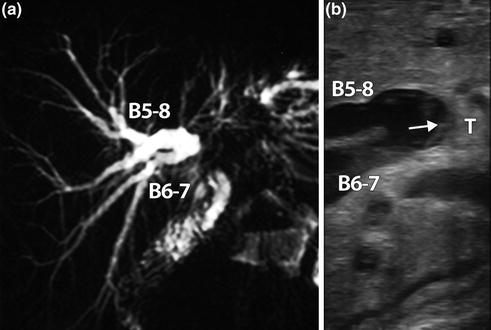
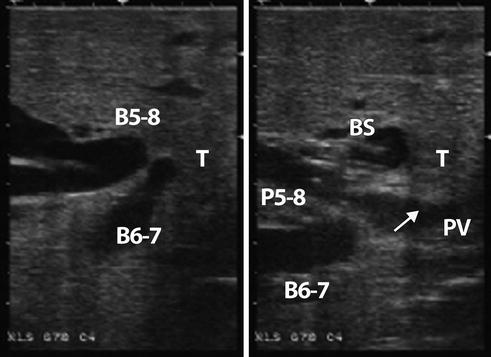

Fig. 12.3
CT scan (a) showing a hepatocellular carcinoma (T) in Sg2-3. Left hepatic vein (LHV) is patent at axial and coronal scans. LUS (b) identified a tumor thrombus (arrow) in the LHV, 15 mm distally to the confluence into inferior vena cava (IVC)

Fig. 12.4
Patient with a peripheral cholangiocarcinoma in Sg7. a CT scan showing a suspected tumor thrombus (white arrow) in Sg7 bile duct. Right anterior sector bile duct is dilated (B5-8). A biliary stent (black arrow) is visible at the bile duct confluence. b The thrombus (white arrow) reaches the common bile duct (CBD) where the biliary stent is visible (black arrow). Sg6 bile duct (B6) is dilated. c LUS longitudinal scan of right posterior bile duct bifurcation. Sg7 bile duct (B7) is filled with the tumor thrombus occluding the confluence with Sg6 bile duct. The biliary stent (S) can be seen in the bile duct confluence (BDC). d LUS cross section of the BDC. The tumor thrombus (TT) occludes B5-8. PVB portal vein bifurcation

Fig. 12.5
Perihilar cholangiocarcinoma. a CT arterial reconstruction. Right hepatic artery is encased by the tumor (arrow) just before the second order division. HA hepatic artery, LHA left hepatic artery, A5-8 right anterior hepatic artery. b LUS confirmed the presence and the extension of right hepatic artery (RHA) infiltration (arrow). A5-8 is free from tumor (T). RPV right portal vein. B5-8 dilated Sg5-8 bile duct

Fig. 12.6
Hilar cholangiocarcinoma. a MR cholangiography showing bilateral biliary dilatation without separation of the right anterior (B5-8) and posterior (B6-7) bile ducts. b LUS showing the tumor (T) and the distance between biliary infiltration (arrow) and B5-8 and B6-7 confluence

Fig. 12.7
LUS of a patient with type IIIA hilar cholangiocarcinoma (T) separating the right anterior (B5-8) and posterior (B6-7) bile ducts. The biliary stent (BS) is visualized in B5-8. The tumor infiltrates (arrow) the portal vein bifurcation (PV). P5-8 portal branch to Sg5-8
12.2 Performance of Laparoscopic Ultrasonography Compared to Intraoperative Open Ultrasonography
Even if encouraging data about LUS has been reported, its reliability in comparison with open IOUS can be assessed only by a direct comparison between the two. In the literature few data are available. In 1996, Cozzi et al. reported a similar sensitivity of the two procedures in detecting hypoechoic artificial lesions in fresh pig livers [14]. In 1997, Tandan et al. analyzed a small series of patients undergoing both laparoscopic and open IOUS before liver resection [15]. Sensitivity and specificity of LUS were good (93 and 100 %, respectively). So far, no further analyses have been published. Recently, the authors conducted a prospective study to clarify these issues [16]. Between September 2009 and March 2011, 65 consecutive patients scheduled for open hepatectomy underwent both laparoscopic and open IOUS. Patients with hilar or gallbladder cholangiocarcinoma were excluded. 119 lesions were diagnosed at preoperative imaging. Despite the high number of preoperative imaging modalities (CT-scan in 100 % of cases, MRI in 67 % and PET-CT in 54 %), laparoscopic IOUS detected 22 additional lesions (+18.5 %) in 14 patients (21.5 %) having a median diameter of 7 mm (2–15). Open IOUS detected two additional lesions (both beside additional nodules detected by LUS), whereas did not confirm four lesions detected by LUS: overall 20 new lesions (+16.8 %) in 10 patients (15.4 %). In comparison with open IOUS, per-lesion LUS sensitivity was 98.6 % and per-patient accuracy was 93.8 %. Agreement between the two procedures for new malignant nodules detection was 99.3 % in per-lesion analysis and 100 % in per-patient one. Considering the detection of vascular and biliary infiltrations similar results have been observed. In comparison with preoperative imaging, new infiltrations were detected by both laparoscopic and open IOUS in six (9.2 %) patients. One additional patient had an infiltration of the left hepatic duct demonstrated by open IOUS, while preoperative imaging and LUS depicted a contact only. Per-lesion sensitivity of laparoscopic versus open IOUS was 96.9 %. Agreement between the two was 99.3 %.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree