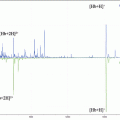
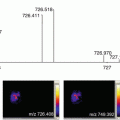
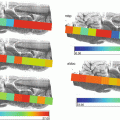
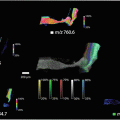
Fig. 1
Schematic diagram on (a) cryosection of barley seed, (b) mounted the section, and the (c) LA-ICP-MS image
3.3 Leaf Material for Imaging
- 1.
Clean and remove any debris, first with a soft clean brush, and then with deionized water, to remove any residues, nutrients, or particles, which could provide false readings (see Note 7 ).
- 2.
Fresh leaves are then placed between tissue paper and pressed between two glass slides.
- 3.
The slides are taped tightly together with masking tape and placed into a freeze drier at −36 °C until they reach a constant mass.
- 4.
Prepare slides by cutting double-sided carbon tape to the correct size and placing onto the slide. Ensure that the size of the leaf does not exceed the ablation area on the stage of the laser ablation system (LA).
- 5.
Carefully un-wrap the tape, and remove the dried leaf.
- 6.
Mount the dried leaves onto the carbon tape on the glass slides, ensuring it is as flat and even as possible. This can be achieved by placing the removed carbon tape cover over the area and pressing firmly with another slide (see Note 5 ).
3.4 LA Reference Material Preparation
- 1.
Obtain fresh matrix matched plant material. It is important that it is as closely matched as possible to ensure accurate results. So, for plant leaves, use leaves from the same type of plant, and for seeds, use the same variety (see Note 6 ).
- 2.
Wash the material with deionized water to remove any residual nutrients or particles and snap freeze using the method in the sample preparation section.
- 3.
Freeze dry to a constant mass to remove any residual water.
- 4.
Homogenize the material by grinding in an electrical grinder or with a pestle and mortar. Pass the resulting powder through a 40-μm sieve to ensure homogeneity.
- 5.
Split the ground plant material into five groups and weigh 1 g ± 0.01 into precleaned falcon tubes. To each tube add the same volume of standard elemental solution, but with increasing concentrations. Always have a blank and only add water to this one. Ensure the powder is saturated and vortex to ensure all powder is dispersed into the solution, as dry clumps can form in the bottom of the tube.
- 6.
Cap the tubes and place onto an oscillating table for 30 min at 200 RPM.
- 7.
Instantly freeze the tubes containing the standards in liquid nitrogen [1], ensuring the whole sample is solid and put into the freezer at −20 °C overnight.
- 8.
Remove from the freezer and place the standards onto a freeze drier at −52 °C and 0.120 mBar until a constant mass is achieved.
- 9.
Add 25% w/w powdered vanillic acid to each of the dried standards. The vanillic acid is used as a binder and it also absorbs strongly at the lasing wavelength of 213 nm. It has been previously documented [2, 3] that vanillic acid not only improves the sensitivity of LA-ICP-MS analysis, but also standardizes the absorptivity of the matrix, which, in turn, improves the quality of the data.
- 10.
To ensure homogeneity and reduce particle size even further, pour the resulting mixture into separate plastic pots with 5 mm ceramic zirconia milling material. Rotary mill for approximately 48 h at 44.6 RPM. This is to improve the homogeneity of the samples.
- 11.
Pass the milled plant material through a sieve to remove the milling material.
- 12.
Press the material into 13 mm pellets using a bench press under vacuum, applying 2 ton of pressure for 1–2 min. Smaller pellets can be produced, but less pressure is required.
- 13.
Digest and quantify the elemental content of the pellets.
3.5 Method Optimization
There are many interchangeable operating parameters that require optimizing in order to produce a fast, high-resolution elemental distribution image. Table 1 summarizes the input parameters on a laser ablation system (see Notes 8 and 9 ).
Table 1
LA input parameters
LA parameter | Typical parameters |
|---|---|
Laser spot size (SS) | 55 μm |
Laser power (LP) | 28% |
Repetition rate (RR) | 20 Hz |
Scan rate/speed (SR) | 95.4 μm/s |
Scan spacing | 55 μm |
Laser warm up time | 20s |
Cell Washout time | 25 s |
ICP-MS acquisition time (AT) | 0.576 s |
Laser Spot Size —The size of this is selected based on the size of the tissue and the features you wish to analyze. A smaller laser spot will give a more detailed image; however, the time taken will increase.
Laser power —It is important to test the tissue prior to analysis and establish the correct laser power. If the energy is too high, there can be too much noise in the signal, thus making the integration of the data difficult. The following steps will help to establish the optimum laser power.
- 1.
Set the laser power at 10% and run an ablation line on the tissue and record the signal.
- 2.
Run fresh ablation lines on the tissue, but increase the power until damage is visible but does not penetrate the material underneath.
- 3.
Repeat this all around the tissue, increasing the laser power incrementally, recording the signal at each level.
- 4.
Continue to increase the power until the signal no longer increases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree