3 months
6 months
9 months
12 months
≥2 years
Type of stent
Uncovered stent (%)
Malapposition
Uncovered stent (%)
Malapposition
Uncovered stent (%)
Malapposition
Uncovered stent (%)
Malapposition
Uncovered stent (%)
Malapposition
BMS
0.1
1.1
0.3–0.6 (OLP: 3.4 %)
0.1–0.17
2.0
0
1.1
0.1
0.3
0
SES
15
16
10.4–25.1 (OLP: 9.6)
1.1–1.3
7.0–11.7
1.5–2.6
5.7–11.6
1.4–1.8
3.2–6.5
0.6–3.3
0.9 (48 months)
PES
3.8
1.5
5.0–10.9 (OLP: 16.5)
1.2–5.7
3.0–4.9
0.6–1.5
5.7–7.1
0.9–3.5
3.7
0.3
ZES-P
0.1
0.2
0.02–1.2 (OLP: 0.37)
0.001–0.2
0.3
0.1
EES
4.7
0.7
2.3–3.2
0.7–2.1
1.6–4.4
0.4–1.8
1.9–5.8
0.1–1.4
ZES-R
6.2
0.7
4.4
0.1
7.4
1.8
BES
21.3
1.9
15.9–21.8
0.5–9.4
4.1
0.1
The incomplete stent coverage was frequently observed in several OCT investigations within 12 months after the 1st generation DES including sirolimus- (SES, Cypher Select™, Cordis, Miami Lake, FL, USA) and paclitaxel- eluting stent (PES, Taxus LiberteTM, Boston Scientific, Natick, MA, USA) (see Table 10.1) [21, 22, 25–27]. These findings support the current guideline that patients treated with 1st-generation DES receive dual anti-platelet therapy (aspirin and clopidogrel) for at least 12 months. To enhance the stent healing process and resolve the safety concerns of 1st generation DES, new-generation DES have been developed with thin strut platform, biocompatible polymer and an optimal dose of anti-proliferative drugs [16, 20, 26, 28–30].
Table 10.1 summarizes the rate of stent strut coverage of various DES and BMS in each period after stent implantation (Fig. 10.1). Comparing SES and PES at 9 months, the frequency of uncovered strut was significantly higher in SES (12.5 ± 15.2 vs. 4.9 ± 7.9 %, p = 0.01) [27]. For the early period group at 3 months, the follow-up OCT data revealed that 15 % of stent struts were uncovered with neointima in SES [25]. Endeavor zotarolimus-eluting stents (E-ZES, EndeavorTM, Medtronic, Santa Rosa, CA, USA) have been developed to resolve the safety issue, by adding a cobalt chromium based thin-strut with a phosphorylcholine biocompatible polymer and a shorter drug elution time (within 2 weeks) [31]. Compared to SES, the coverage of Endeavor ZES was almost complete irrespective of clinical presentation at 6–9 months [20, 22, 26]. The ODESSA (Optical Coherence Tomography for Drug-Eluting Stent Safety) study showed that ZES had a significantly lower incidence of uncovered stent struts than SES at both the overlapping and non-overlapping sites (0.02 vs. 5.8 % in overlapping sites and 0.01 vs. 6.0 % in non-overlapping sites) [22]. Furthermore, our observation showed that most stent struts (99.9 %) in Endeavor ZES were covered with neointima and were well-apposed at 3 months [16]. Other 2nd generation DES were developed to improve the safety concerns while maintaining the efficacy of 1st generation DES. Everolimus-eluting stents (EES, XienceTM, Abbott Vascular, Santa Clara, CA, USA) and Resolute zotarolimus-eluting stents (R-ZES, ResoluteTM, Medtronic, Santa Rosa, CA, USA) are a cobalt chromium-based thin strut stent with a biocompatible polymer (Biolinx polymer in R-ZES and Fluorinated copolymer in EES) and a reduced dosage of drug [29]. EES and R-ZES also showed higher rates of strut coverage than SES at 9 months but were comparable [29]. The early stent strut coverage based on serial OCT evaluations was comparable between ZES-R and EES at 3 months (EES vs. ZES-R: 4.7 ± 5.1 vs. 6.2 ± 6.9 %, p = 0.62) [30]. The presence of permanent polymers of 1st generation DES is thought to be responsible for a hypersensitivity reaction which might cause incomplete re-endothelialization [32]. To overcome these issues, the development of new-generation DES utilizing a biodegradable polymer has been introduced. Biolimus-eluting stents (BES, NoboriTM, Terumo Corporation, Tokyo, Japan or BioMatrixTM, Biosensors Interventional, Singapore) have the unique property of a biodegradable polymer with an abluminal drug coating. A recent OCT study comparing SES demonstrated that OCT-defined coverage of BES was greater than that of SES at 6–9 months [33]. Serial assessment of stent strut coverage and malapposition may provide further insight into understanding clinical situations (Fig. 10.2). In serial observations of BES and SES between 3 and 12 months, both BES and SES showed a high percentage of incomplete strut coverage without any significant changes at 3 months. However, BES showed a significantly lower percentage of uncovered struts at 12 months compared to SES [34]. This was achieved by improved strut coverage from 3 to 12 months. Another study assessed the serial changes between 9 and 2 years and showed that the percentage of uncovered struts significantly decreased from 4.4 % at 9 months to 2.3 % at 2 years (p < 0.001) [35]. Completely covered lesions were also more frequently observed at 2 years (44.7–59.2 %, p = 0.07), although approximately half of the stented lesions were still not completely covered during extended follow-up [35]. Based on these observations, strut thickness may be a crucial factor to stent struts coverage during the early period (3–6 months).
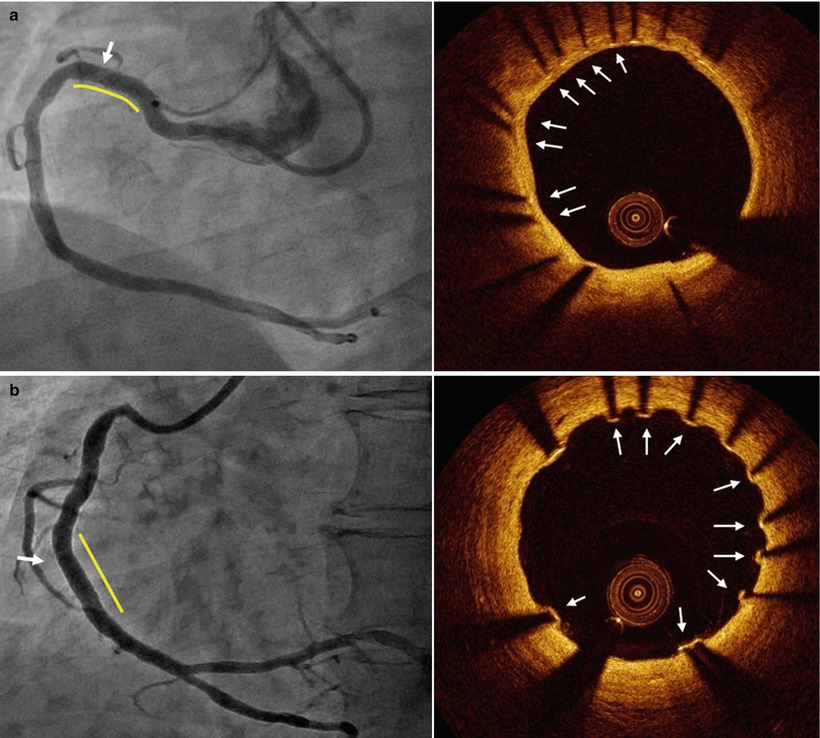
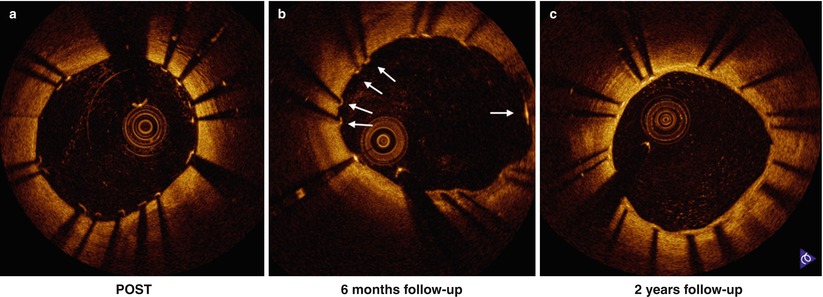

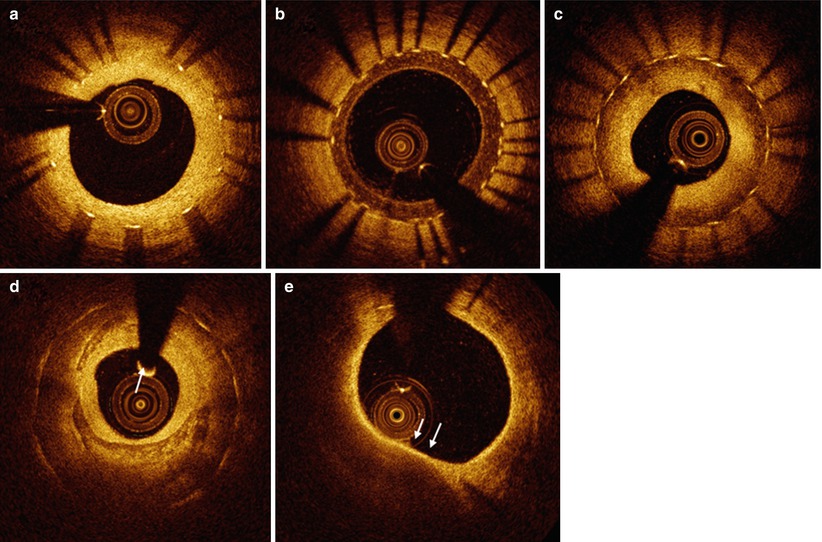
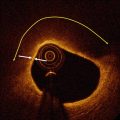
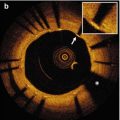
Fig. 10.1
Representative images of stent strut coverage. Optical coherence tomography showed the complete stent strut coverage at 12 months after everolimus eluting stent implantation (arrows) (a) and incomplete stent strut coverage with frequent detected uncovered struts at 3 months after biolimus eluting stent (arrows) (b)

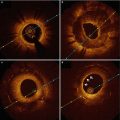
Fig. 10.2
Serial change of stent strut coverage at post intervention (a), 6 months (b) and 2 years (c). Some struts were not covered at 6 months (b, white arrow) and stent struts coverage completed at 2 years (c)
For the clinical manifestation, the pattern of neointimal coverage after DES implantations were different between acute coronary syndrome (ACS) including ST elevation myocardial infarction (STEMI) and stable angina pectoris (SAP), and variable among the DES types between two groups [36–38]. Therefore, the current study suggests that the vascular response after DES implantation might be influenced by both clinical presentation and the type of DES.
The next task is in the application of these findings to a real clinical situation. A recent autopsy study revealed that delayed and incomplete neointimal coverage of DES was associated with occurrence of late stent thrombosis; the odds ratio for stent thrombosis in a stent with a ratio of uncovered to total stent struts per section >30 % was 9.0 (95 % confidence interval, 3.5–22) [10]. In a case-control study in which OCT was performed at the onset of stent thrombosis, the length of an uncovered stent strut segment was significantly associated with stent thrombosis [39]. Another cross-sectional analysis with 18 patients that experienced very late stent thrombosis showed that for 14 stents which developed very late stent thrombosis (VLST) without neointimal rupture, uncovered struts were observed in 9 stents (64.3 %) [40]. Based on these observations, strut coverage assessed by OCT may be a surrogate marker for predicting serious cardiovascular events in daily clinical practice. A recent OCT study suggested that a greater percentage of uncovered struts (a cut-off value of ≥5.9 % uncovered struts), as assessed by OCT at the 6–18 months follow-up, in asymptomatic DES-treated patients might predict the increase in major adverse cardiac events which is very relevant to stent safety in the future [41].
Current OCT analysis with conventional methods may have a challenge to compare the change of strut coverage and malapposition at the strut level during serial follow-up at different time points. Recently a contour plot OCT analysis was proposed as a possible method of assessing individual stent struts at the strut level practically; this comprehensive monitoring of stent strut status at different time points would then provide useful information regarding vascular healing status after DES implantation (Fig. 10.3) [42, 43].
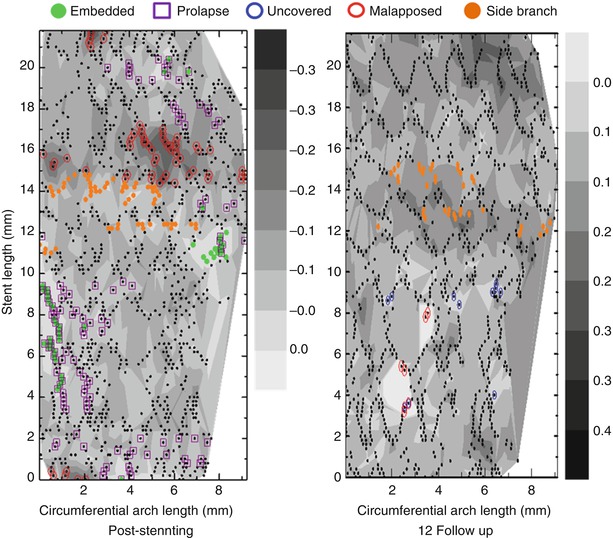

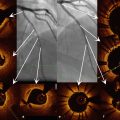
Fig. 10.3
Representative images of contour plot images post-intervention and at follow-up. Using a contour map OCT analysis, each stent struts can be tracked appropriately
10.2 Malapposition
During follow-up, the definition of malapposed struts that is applied is the same that is used immediately post stent implantation, namely one distinguishing the various types of coronary stents based on differences in the thickness of struts and the polymer (Fig. 10.4). The following are the criteria of malapposed struts for several DES: ≥160 μm for sirolimus-eluting stents (Cypher Select™, Cordis, Miami Lake, FL, USA), ≥130 μm for paclitaxel-eluting stents (Taxus LiberteTM, Boston Scientific, Natick, MA, USA), ≥110 μm for zotarolimus-eluting stents (EndeavorTM and ResoluteTM, Medtronic, Santa Rosa, CA, USA), ≥100 μm for everolimus-eluting stents (XienceTM, Abbott Vascular, Santa Clara, CA, USA) and ≥130 μm for biolimus-eluting stents (NoboriTM, Terumo Corporation, Tokyo, Japan or BioMatrixTM, Biosensors Interventional, Singapore) [26, 27, 29, 33, 44, 45].
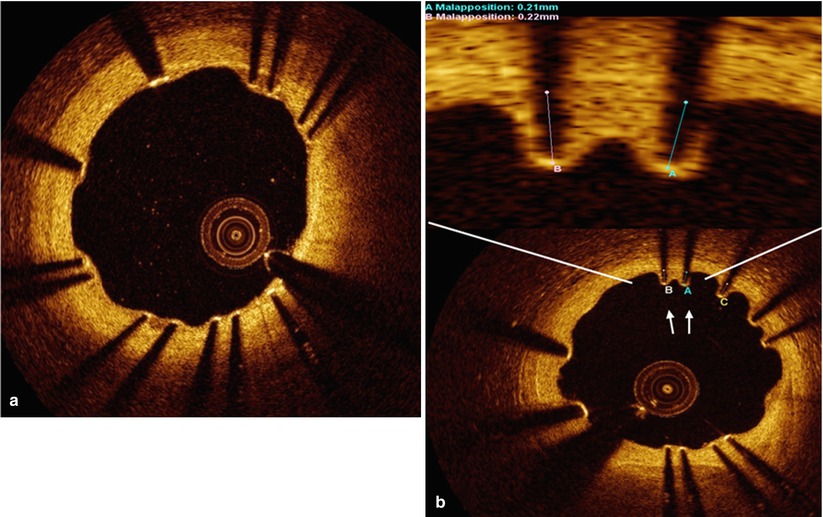

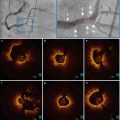
Fig. 10.4
Representative images of stent strut apposition. Optical coherence tomography showed the well apposed stent strut with complete coverage at 6 months after biolimus eluting stent implantation (a) and malapposition with uncovered struts in part of stent at 6 months after resolute zotarolimus eluting stent (arrow) (b)
As neointimal formation proceeds after stent implantation, the rate of malapposed struts significantly decreases when the detachment distance between the stent strut and vessel wall is within a certain distance, which is different depending on the type of stent [46–48]. A previous OCT report demonstrated that the percentage of malapposed struts remarkably decreased from 12.2 ± 11.0 % post-intervention to 1.0 ± 2.2 % at the 6 month follow-up [48]. However, during serial follow-ups between 2 and 4 years, the one study with SES reported a significant increase in the percentage of malapposed struts from 2.2 % at 2 years to 3.0 % at 4 years (p = 0.044) in SES and our study revealed similar findings including 1st and 2nd generation DES that malapposed struts were similarly detected between 9 months and 2 years (0.6–0.9 %) [49, 50]. These findings were usually observed in 1st generation DES and could be possibly explained by long-term vascular inflammation. Malapposed struts could be classified into persistent (persistently malapposed after an index procedure) and late-acquired malapposition (newly detected at follow-up). Recent follow-up OCT examinations indicated that the percentage of late-persistent malapposed struts was 2.5 ± 3.6 %, and most (72 %) late-persistent stent malappositions were located within the edges of the stents. For late acquired malapposition, the percentage of late-acquired malapposed struts was 3.8 ± 4.5 %, which were predominantly distributed within stent bodies (61 %) rather than stent edges (39 %). The plaque/thrombus prolapse was observed more frequently by poststent OCT examinations in patients with late-acquired stent malapposition lesions compared with those who did not have late-acquired stent malapposition lesions. In their 2013 paper Kawamori et al. suggest a the strut-to-vessel distance of ≤260 m on post-stenting OCT images as a cut-off value for resolved malapposed struts (sensitivity of 89.3 % and a specificity of 83.7 %) for 1st generation DES (SES or PES) [51]. In their more recent paper, Foin et al. studied the 2nd generation DESs and drug eluting balloon in combination with a bare metal stent, demonstrating that malapposed segments with detachment from the vessel wall of <100 m at baseline showed complete strut coverage at follow-up, whereas segments with a maximal malapposed detachment distance of 100–300 m and >300 μm had 6.1 and 15.7 % of their struts still uncovered at follow-up, respectively (P < 0.001) [46]. Additionally, in another study, coverage of malapposed struts was delayed with respect to well-apposed struts at the 9–13 months post DES implantation follow-up [52]. Although overall malapposed struts were positively correlated with uncovered struts, a subgroup of lesions with minimal stent malapposition showed similar rates of uncovered struts to those without malapposition [53, 54]. The coverage of malapposed struts was also affected by DES type, namely between the first- and new-generation DES in the initial clinical presentation [55]. The incidence of malapposed stent struts was significantly higher in ACS at 9 months (2.2 ± 5.6 vs 0.5 ± 2.0 %, p = 0.02) [36]. Especially in STEMI, the incidence of malapposed struts was significantly higher in SES and PES than ZES (SES vs PES vs ZES: 4.0 ± 8.2 % vs 2.1 ± 4.5 % vs 0 ± 0 %, p = 0.001) [37].
The next logical step was then to identify the clinical implication of stent malapposition during follow-up. A recent OCT study reported that severe stenosis, calcified lesions, and longer stent length were predictors for acute stent malapposition. Predictors of late-persistent stent malapposition were the presence of acute stent malapposition within the stent edge and a larger volume of acute stent malapposition. Late-acquired stent malapposition was associated with plaque/thrombus prolapse detected on poststent OCT images. Long-term clinical outcomes of late stent malapposition detected by OCT were favorable [44]. However (Using “but” at the beginning of a sentence is strongly discouraged), this investigation was a cross-sectional study and moreover, was limited by the small number of clinical events in this study.
10.3 Neointimal Characteristics
Pathological studies have demonstrated that neointima within a stent are comprised of various tissue components including collagen, proteoglycan, smooth muscle, fibrin, or thrombus [56, 57]. Previous imaging modalities, such as angiography and intravascular ultrasound, have been limited by their low resolution, in detecting crucial neointimal characteristics. However, intravascular OCT, with its higher resolution, is useful for both the qualitative as well as quantitative evaluation of neointimal tissue [35, 58]. Recent OCT studies have reported differential morphological characteristics of neointimal tissue, which were well-correlated with histological findings [7, 59]. The stented segments at the minimal lumen CSA were assessed qualitatively to characterize the neointimal tissue as (1) homogeneous neointima, a uniform signal-rich band without focal variation or attenuation; (2) heterogeneous neointima, focally changing optical properties and various backscattering patterns; and (3) layered neointima, layers with different optical properties, namely an adluminal high scattering layer and an abluminal low scattering layer (Fig. 10.5) [7, 35, 58]. Heterogeneous neointima were frequently detected in 21.7 % of DES-treated lesions in the Yonsei OCT registry. The occurrence of neointimal tissue within this group was significantly associated with both old age and initial clinical presentation of ACS [60].