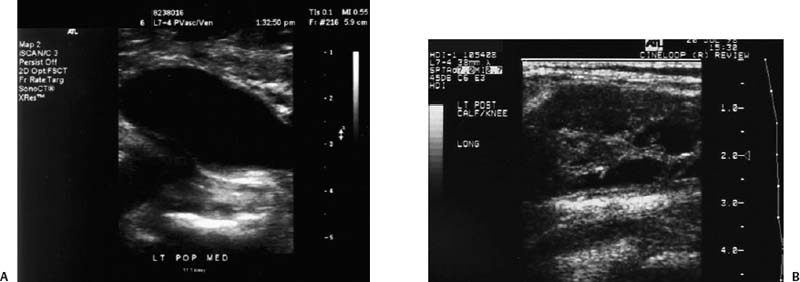
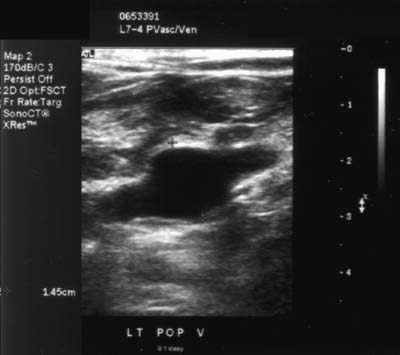
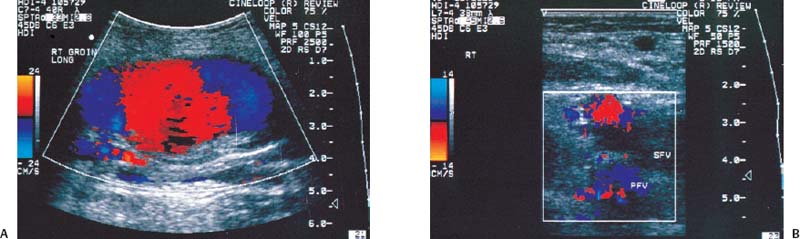
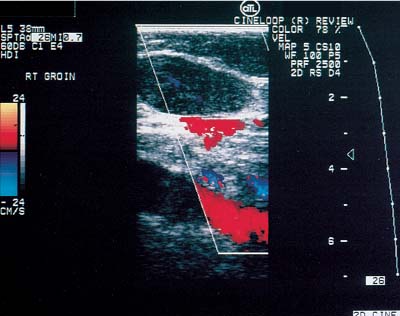
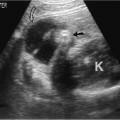
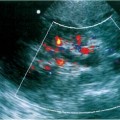
12 Leg Swelling with Pain or Edema Patients who present with leg swelling with pain or edema are of great clinical concern because they are at risk for having acute deep venous thrombosis (DVT). Acute DVT is an important public health problem because it affects over 20 million individuals yearly in the United States. Estimates of the incidence of DVT range from between 100 and 180 per 100,000 persons per year.1 DVT occurs in both sedimentary outpatients as well as hospital inpatients, although rarely in children. Among the predisposing factors are prolonged congestive heart failure, pelvic and lower extremity surgery, pregnancy, obesity, inactivity, airplane travel, coagulopathy, and paraplegia.2 The diagnosis of DVT has other significant implications as well. It has been known for more than a century that DVT may be a presenting feature of an occult neoplasm. Recently, a definite association between DVT and a subsequent clinically occult cancer has been shown, particularly in the first 6 to 12 months of follow-up.3 However, in the study by Sorensen et al 40% of the patients diagnosed with cancer within 1 year of the diagnosis of DVT had distant metastases. As a result, it is uncertain whether an extensive search for an occult neoplasm would be cost-effective or warranted because early diagnosis may not change outcomes. The types of cancers most strongly associated with DVT in this Swedish study of more than 15,000 patients were cancer of the pancreas, ovary, liver (primary hepatic cancer), and brain.3 Figure 12–1 (A) Oblique view of a large sonolucent mass located in the popliteal fossa separate and apart from the artery and vein on sagittal color flow Doppler imaging. This is consistent with a Baker’s cyst. (B) Oblique image of a complex mass in the popliteal space of another patient. This mass is separate and medial to the artery and vein and is characteristic of a ruptured Baker’s cyst. Presently, it is important to diagnose acute DVT because of its relationship with acute pulmonary embolism. The presence of DVT does not equate with pulmonary embolism, but detecting DVT may prevent pulmonary embolism from developing. It is reported that when significant acute venous thrombosis is diagnosed but not treated, pulmonary embolism is likely to occur in up to 50% of the cases.4 More importantly, it is believed that in up to 30% of the episodes of pulmonary embolism the outcome is death.4 Pulmonary embolism is reported to be the cause of death in more than 10,000 hospitalized patients each year in the United States.5 Mortality can be significantly reduced when acute DVT is treated with anticoagulation. Because an estimated 90% of pulmonary emboli arise from the lower extremities, there is a great clinical need to accurately assess the venous system of the lower extremities when there is clinical suspicion of acute DVT. Patients who present with the clinical symptomatology of leg swelling with pain or edema certainly are at risk for acute DVT. However, the clinical accuracy of diagnosing this entity is known to be very poor. Every clinical sign attributed to DVT has been statistically analyzed and found to be of no value in reliably determining the presence or absence of DVT.6 The location of signs and symptoms of pain or swelling is usually unrelated to the extent or location of clot within the veins. Symptoms localized to the calf may have an etiology in the femoral veins, and thigh pain may be related to occlusion of calf veins.7 The specificity for clinical diagnosis is low because the symptomatology associated with acute DVT can have, among other causes, a musculoskeletal or lymphatic basis. Furthermore, asymptomatic DVT can commonly occur, and the sequelae can even be severe enough to cause death by pulmonary embolism. In patients who present with bilateral leg symptoms, in the absence of significant risk factors (such as malignancy, paralysis, bed rest of more than 3 days, major surgery, strong family history of DVT, or leg, thigh, or calf swelling)8 the first assumption should be that the etiology is cardiac disease or chronic peripheral vascular disease.9 However, in patients with risk factors, the possibility of bilateral clot must be seriously considered, and a bilateral ultrasound examination is warranted. D-dimer has emerged as a clinically useful serological test. D-dimer represents a breakdown of the cross-linked fibrin clot. It is elevated in almost all patients with acute DVT, having a sensitivity of 97%, but it is nonspecific (less than 50%). D-dimer is also elevated in patients who have had recent surgery, trauma, sepsis, and malignancy.1 However, it is very useful in excluding DVT having a negative predictive value of 97%.10 In certain patient groups, Ddimer combined with clinical assessment has been shown to safely reduce or eliminate the need for noninvasive testing.10 The differential diagnosis for the symptoms of leg swelling with pain or edema includes acute DVT, Baker’s (popliteal) cyst, cellulitis, lymphedema, chronic venous insufficiency, superficial thrombophlebitis, popliteal venous aneurysm, popliteal artery aneurysm, iliac artery aneurysm, femoral artery pseudoaneurysm, enlarged lymph nodes extrinsically compressing the veins, heterotopic ossification, hematoma, muscular tears, and diabetic muscle infarction.7,11–19 The appropriate use of imaging studies, and, in particular, the appropriate use of ultrasound, enables us to distinguish which of these clinical entities is present. Baker’s cysts appear as sonolucent or complex masses more commonly medial than lateral. When they rupture, the surface margins become irregular (Fig. 12–1A,B). They appear separate and distinct from the popliteal artery or vein. The normal popliteal artery measures less than 1 cm. Veins and arteries should taper normally, and an outpouching would suggest aneurysmal dilatation (Fig. 12–2). Frequently, thrombus may surround the residual lumen of an arterial aneurysm. Aneurysms or pseudoaneurysms, owing to their expanded size, can compress the venous structures that surround them, resulting in swelling or edema of the distal extremity. The visualization of a concomitant vascular abnormality should, therefore, not preclude further evaluation of the more distal venous structures because the resultant stasis may be a predisposing factor to the development of DVT (Fig. 12–3). Patients with superficial thrombophlebitis have an increased risk of progressing to DVT. In one report, 11% of patients who initially had isolated superficial venous thrombus progressed to DVT.20 In another study, 23% of patients with superficial thrombophlebitis had occult DVT of the lower extremities.21 As a result, if superficial venous thrombosis is noted, then the deep venous system should be carefully studied. Similarly, enlarged lymph nodes can also extrinsically compress adjacent venous structures, resulting in lower extremity symptoms (Fig. 12–4). This has been noted in patients with acquired immunodeficiency syndrome (AIDS). On rare occasions, pain or swelling of the lower extremity may be the presenting symptom of lymphoma. Lymph nodes appear separate from the vascular structures and are easily distinguished using color flow Doppler imaging (CFDI). Hematomas are also separate and distinct from the arteries and veins and, depending on their age, appear as either complex or sonolucent masses. Figure 12–2 Sagittal color flow Doppler imaging of a 1.5 cm popliteal venous aneurysm. Figure 12–3 (A) Large pseudoaneurysm of the right common femoral artery (CFA) postcardiac catheterization compressing the common femoral vein (CFV). (B) Transverse image showing the more expanded hypoechoic noncompressible proximal superficial femoral vein (SFV) consistent with acute thrombus. PFV, profunda femoral vein. Tests to evaluate the lower extremities include contrast venography, venous ultrasound including CFDI and Doppler spectral analysis, impedance plethysmography (IPG), various radionuclide approaches, magnetic resonance (MR) venography, computed tomography (CT), non-imaging continuous wave Doppler, and thermography. All evaluation methods were studied by the Cardiovascular Appropriateness Panel of the American College of Radiology (ACR), and each of these methods was rated for appropriateness in 1995 and reassessed in 1999 (Table 12–1).22 Contrast venography has been the gold standard by which other examination methods have been rated; however, in 5 to 10% of patients it may not give a reliable result, and because it also requires the use of intravenous contrast with the associated risks of renal failure, extravasation, chemical-induced thrombophlebitis, and idiopathic contrast reactions, its appropriateness rating was only intermediate. In contrast, duplex Doppler compression ultrasound received the highest possible rating and, therefore, now should be used as the study of choice to evaluate symptomatic patients. Figure 12–4 Solid mass density in the right inguinal area separate and apart from the common femoral artery and common femoral vein. On biopsy this mass of nodes was diagnosed as non-Hodgkin’s lymphoma. The panel reported also that thermography and nonimaging continuous wave Doppler analysis were inappropriate and should not be performed. IPG was given an intermediate appropriateness rating as was MR venography. The accuracy of IPG is reported to be close to 90% for DVT above the knees, but it requires meticulous technique. Heijboer et al reported that serial ultrasonography is a more accurate means of detecting DVT than is IPG. An abnormal IPG had a positive predictive value of 83% for DVT compared with a 94% rate for an ultrasound abnormality in patients with DVT.23 Radionuclide venography was given a lower intermediate rating. The accuracy of detecting large obstructive thrombi is reported to be close to 90%. Another approach that can be used and appears best to diagnose DVT below the knee or in the lower thigh is the active uptake of radionuclide-labeled fibrinogen, platelets, peptides, fibrin, and plasmin by thrombi. New imaging agents may also be helpful in differentiating acute recurrent from chronic DVT. CT is most useful for imaging thrombosis related to the iliac veins and is not particularly suitable for studying the femoropopliteal veins. It is also valuable in identifying sources of extrinsic compression of the iliac veins. Recently, CT venography has been recommended as a quick and safe means to evaluate the deep venous system after multidetector row CT (MDCT) angiography of the pulmonary arteries (within the same examination), particularly if the patient is clinically unstable and requires immediate diagnosis.24,25 This methodology has a reported sensitivity ranging between 71 and 100%, specificity between 93 and 97%, positive predictive value between 53 and 92%, and negative predictive value between 92 and 97%.24,26,27 However, the estimated median cumulative effective radiation dose is 8.26 mSv [compared with 2.5 mSv for CT angiography (CTA) of pulmonary arteries] for all patients with an effective gonadal dose of 3.87 mSv. This dose is more than 300% of the median effective dose for CTA of the pulmonary arteries (2.5 mSv). This dose must be significantly reduced before it could become a routine clinical method to evaluate for DVT in clinically stable patients, particularly for those who are young.
Accuracy of Presentation in Diagnosing Deep Venous Thrombosis
Accuracy of D-Dimer Test in Diagnosing Deep Venous Thrombosis
Differential Diagnosis
Diagnostic Imaging Evaluation
Clinical Condition | ||
Radiologic Procedure | Appropriateness Rating | Comments |
US, lower extremity, duplex Doppler compression | 9 |
|
MRI, venography, lower extremity | 6 | Demonstrated to be useful, but insufficient supporting data so far. |
INV, venography, pelvis | 6 | When other studies equivocal or an intervention is planned. |
CT, pelvis, with contrast | 6 | As an adjunct to CTPA done for suspected PE. |
INV, venography, lower extremity | 5 | When other studies equivocal or an intervention is planned. |
CT, venography, lower extremity (following arm injection) | 5 | As an adjunct to CTPA done for suspected PE. |
NM, venography (MAA), lower extremity | 3 |
|
IPG, lower extremity | 2 |
|
X-ray, lower extremity | 2 |
|
Continuous wave Doppler (nonimaging), lower extremity | 1 |
|
Appropriateness Criteria Scale:
1 = Least appropriate, 9 = Most appropriate
INV = interventional venography
MR venography has also been suggested as a useful technique to detect DVT in the lower extremities.28,29 It appears to be particularly useful in detecting femoral and iliac vein thrombi and may be particularly valuable in determining the proximal extent of disease in these vessels. Newer techniques such as venous enhanced subtracted peak arterial (VESPA) MR venography,30 flow-independent MR venography,5 and direct thrombus MR imaging,31,32 may allow MR to play a more significant role in DVT imaging in the future.
One major limitation of both MR and CT is the significant increase in cost relative to ultrasound. Presently, both may be most useful for evaluating the more proximal venous structures when a satisfactory study is not achieved with ultrasound. But when considering the overall sensitivities, specificities, and costs of the various testing options, ultrasound remains the screening test of choice for lower extremity DVT.
Ultrasound Imaging
Evaluating patients with symptoms of leg swelling with pain or edema should involve using all the capabilities of ultrasound when available, including Doppler spectral analysis, CFDI, transducer compression, and high-resolution B-mode imaging. The examination should integrate all these methodologies into the complete study of the venous structures of the lower extremity. Extended field of view sonography, a new option of some machines, may be useful in demonstrating more easily the full extent of disease. The use of contrast agents and harmonic imaging may be helpful, in the future, in cases in which the complete occlusion of vessels is uncertain.
Normal Findings
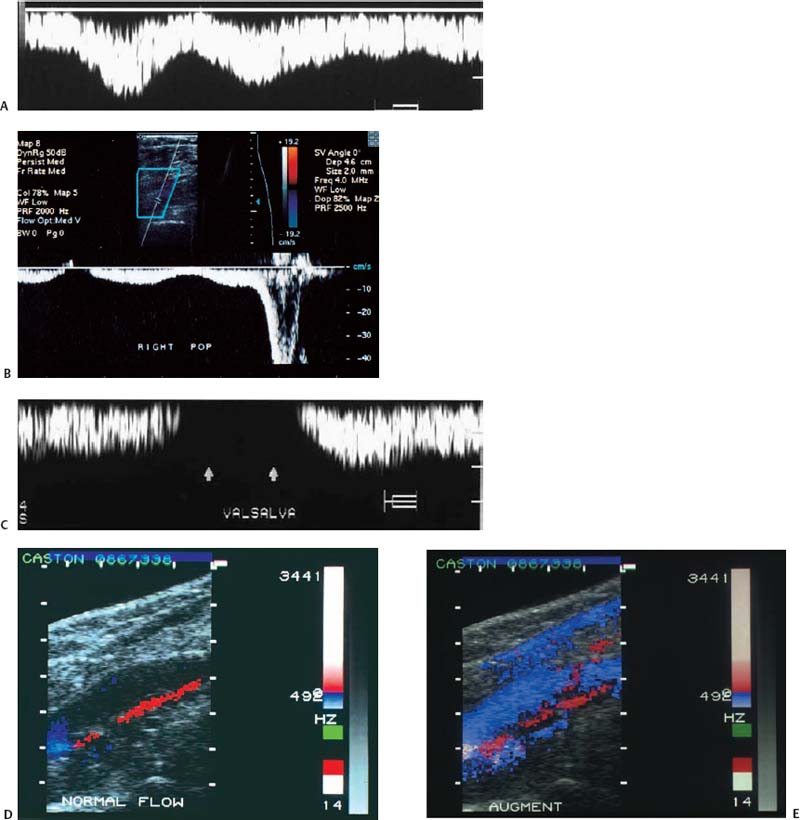
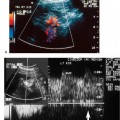
With Doppler spectral analysis and CFDI, three parameters of normal lower extremity veins can be identified: respiratory phasicity, spontaneous flow, and augmentation (Fig. 12–5A–E). During the respiratory cycle, the change in venous flow is termed respiratory phasicity (Fig. 12–5A). During inspiration, an increase in intraabdominal pressure causes compression of the inferior vena cava and a decrease in the Doppler signal. In expiration, there is an increase in visualized flow and in the Doppler spectral analysis signal (Fig. 12–5B). The Valsalva maneuver leads to stasis and venous dilatation. The normal physiological response of the femoral vein is a 50 to 200% increase in diameter. At the end of the Valsalva maneuver, augmented venous flow normally occurs (Fig. 12–5C). In a normal venous system, augmentation also occurs with distal mechanical compression (Fig. 12–5B,D,E). Spontaneous venous flow is easily detected in the large leg vessels, but is frequently not seen in some of the smaller calf vessels. Augmentation will frequently be required to prove patency.
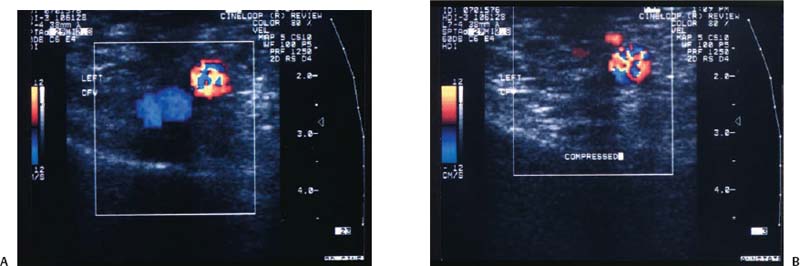
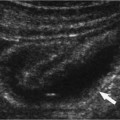
A normal vein also demonstrates compressibility, has unidirectional flow, and is free of internal echoes. Compressibility is best demonstrated when the transducer is held in the transverse position relati ve to the vein because the transducer will not roll off the vessel (Fig. 12–6A,B). An adequate amount of pressure sufficient to dimple the overlying skin should be applied with the transducer to visualize the normal sonographic finding of a collapsed venous lumen. Unidirectional flow is best identified with CFDI.
Figure 12–5 (A) Normal parameters of lower extremity veins include respiratory phasicity, (B) augmentation following distal compression, and (C) absence of flow during the Valsalva maneuver and then slight augmentation following the maneuver. These are demonstrated with Doppler spectral analysis. (D) Augmentation can also be demonstrated with color flow Doppler imaging following (E) distal compression.
Technique
The choice of transducers for studying the lower extremities depends on the patient’s body habitus and the depth of the vessel being studied. For the average patient, a 5 to 10 MHz linear transducer is most appropriate. Vessels ranging in size from 1 mm to slightly over 1 cm can be clearly visualized over a depth of 6 cm. For large-body-habitus patients, transducers of lower MHz can be used. Linear array transducers permit long segments of vessels to be imaged more rapidly and are therefore preferred. Transducers will frequently need to be switched when the examiner moves from the inguinal region to the thicker thigh or the calf veins.
Figure 12–6 (A) Transverse color flow Doppler imaging demonstrating the normal common femoral vein (CFV–blue) and the common femoral artery (CFA–red) without and (B) with compression. Note the collapse of the normal vein.
Vessels that can be studied include the common femoral veins; saphenous veins; superficial femoral veins (SFVs); popliteal veins; calf veins, including the anterior tibial, posterior tibial, and peroneal veins; and if possible, the iliac veins (Fig. 12–7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree