, Maria Custódia Machado Ribeiro2 and Bruno Beber Machado3
(1)
Hospital da Criança de Brasília – Jose Alencar, Clínica Vila Rica, Brazília, Brazil
(2)
Hospital da Criança de Brasília – Jose Alencar, Hospital de Base do Distrito Federal, Brasília, Brazil
(3)
Clínica Radiológica Med Imagem, Unimed Sul Capixaba, Santa Casa de Misericordia de, Cachoeiro de Itapemirim, Brazil
Abstract
Legg-Calvé-Perthes disease (LCPD) is an idiopathic form of avascular necrosis of the immature proximal femoral epiphysis. As it occurs in the growing skeleton, there are peculiarities that distinguish it from the adult type of avascular necrosis of the femoral head. It is important to keep in mind that this epiphysis bears a significant remodeling potential in skeletally immature patients, which can correct architectural changes caused by ischemia; the younger the patient, the greater is this ability. Nevertheless, abnormal epiphyseal development and deformation of the femoral head do occur in cases of poor evolution. The cascade of phenomena that occur after bone necrosis is paralleled by the evolution of imaging findings. This chapter will provide an overview of the role of imaging in the assessment of LCPD.
7.1 Introduction
Legg-Calvé-Perthes disease (LCPD) is an idiopathic form of avascular necrosis of the immature proximal femoral epiphysis. As it occurs in the growing skeleton, there are peculiarities that distinguish it from the adult type of avascular necrosis of the femoral head. It is important to keep in mind that this epiphysis bears a significant remodeling potential in skeletally immature patients, which can correct architectural changes caused by ischemia; the younger the patient, the greater is this ability. Nevertheless, abnormal epiphyseal development and deformation of the femoral head do occur in cases of poor evolution. The cascade of phenomena that follows bone necrosis is paralleled by the evolution of imaging findings. This chapter will provide an overview of the role of imaging in the assessment of LCPD.
7.2 Clinical Aspects
LCPD is more common in white males, with an incidence peak around 4–8 years, and the disease is bilateral in 10–20 % of the patients. Symptomatic children present insidious hip pain and intermittent limp, which are exacerbated by physical activity, as well as limited abduction and internal rotation of the affected hip. In general, the younger the patient and the smaller the extent of the epiphyseal necrosis, the better is the prognosis. Similarly, prognosis for older children and/or patients with large necrotic areas tends to be poor. As the prognosis is better in the most affected age group, the majority of the patients have good evolution.
Considering its idiopathic nature, LCPD is a diagnosis of exclusion. Thus, potential causes of avascular necrosis must be ruled out, such as systemic diseases, complications of drug therapy (mostly systemic corticosteroids), and other causes of impaired epiphyseal blood supply (e.g., vasculitis, radiation therapy and surgery). Infectious and noninfectious arthritides, slipped capital femoral epiphysis, traumatic injuries, and transient synovitis must be considered in the differential diagnosis. Even though epiphyseal dysplasias can closely resemble LCPD on imaging, dysplastic abnormalities are usually bilateral, simultaneous, and symmetric, with flattening/fragmentation of the femoral heads (and other epiphyses as well) and delayed maturation of the secondary ossification centers (see Chap. 12).
7.3 Imaging
7.3.1 Radiographs
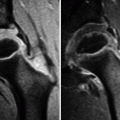
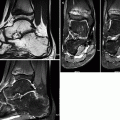
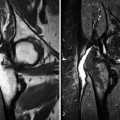
Imaging investigation usually starts with radiographs of both hips (anteroposterior and lateral views). Radiographs are most often normal during the initial phase of LCPD, though subtle epiphyseal osteoporosis may be occasionally present. During the condensation phase, there is an increase in the epiphyseal density due to bone apposition and thickening of the trabeculae (Fig. 7.1). The classic subchondral fracture – Caffey’s sign or “crescent sign” – appears as a linear lucency parallel to the articular surface of the femoral head, often associated with epiphyseal flattening (Figs. 7.1, 7.2, and 7.3). Widening of the joint space may also be found, related to cartilaginous thickening, as the superficial cartilage is nourished via diffusion from the synovial fluid and does not suffer from ischemia at this stage. The so-called metaphyseal reaction, characterized by the appearance of round lucencies (commonly referred to as “cysts”) along the physis, also begins in the condensation phase (Figs. 7.3, 7.4, and 7.5), as well as bone remodeling of the femoral neck, which becomes progressively shortened and broadened; the growth plate may appear wavy or cupped. In the following stage, referred to as the fragmentation phase, the ossified portion of the epiphysis becomes fragmented and heterogeneous, occasionally exhibiting a central sequestrum (Fig. 7.6). There is also progression of the metaphyseal reaction and of the bone remodeling of the femoral neck (Figs. 7.6, 7.7, and 7.8). Widening of the joint space becomes even more marked, and acetabular deformity may occur, with lateral protrusion of the femoral head (Figs. 7.6 and 7.8). Progressive restoration of bone density and reossification of the ischemic epiphysis occurs with healing, during the reparative phase (Fig. 7.9). Definitive deformation and loss of the spherical shape of the epiphysis are found in cases of poor evolution, as well as varus deformity of the femoral neck, with eventual onset of secondary osteoarthritis (Fig. 7.10). As stated above, epiphyseal dysplasias are usually bilateral and symmetric, with simultaneous involvement of other joints, features that allow them to be distinguished from LCPD (Fig. 7.11).
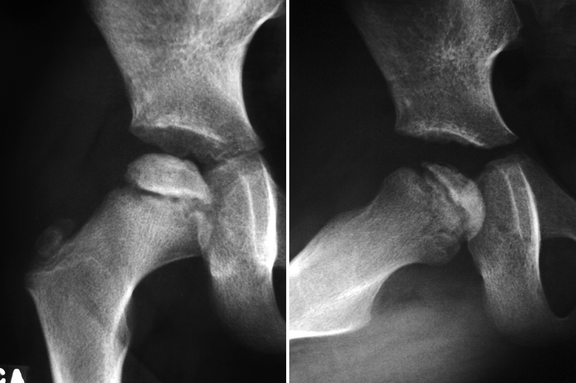
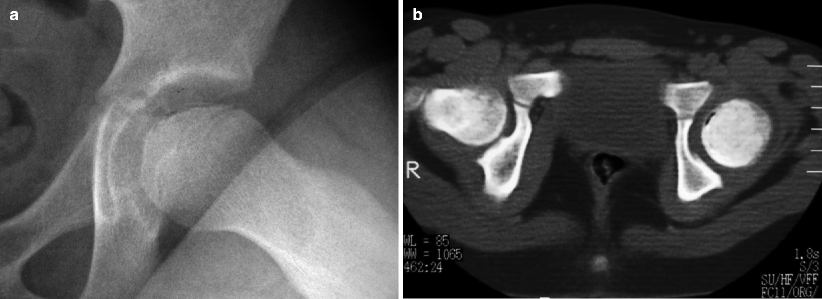
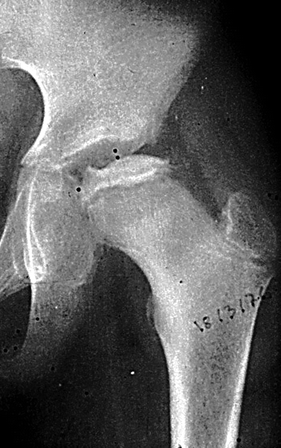
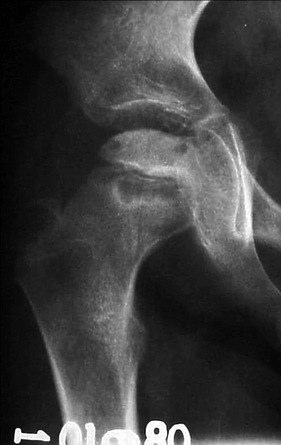
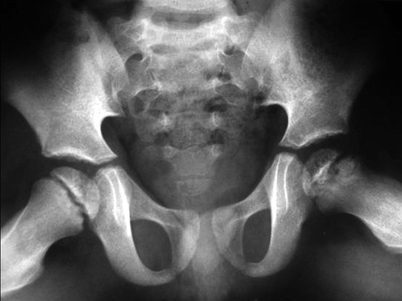
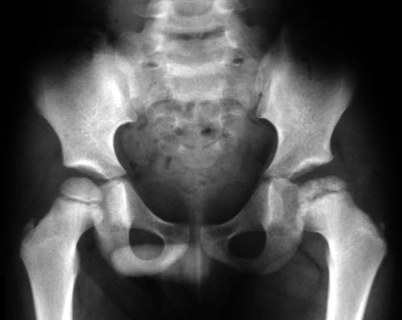
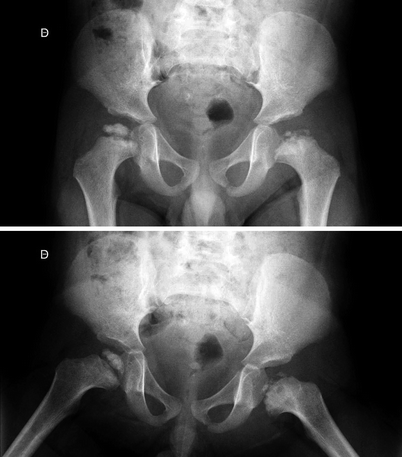
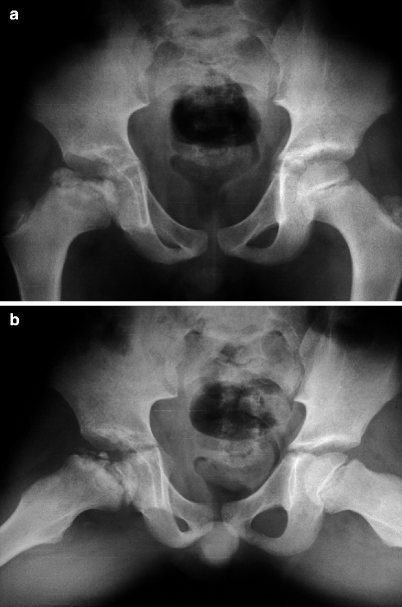
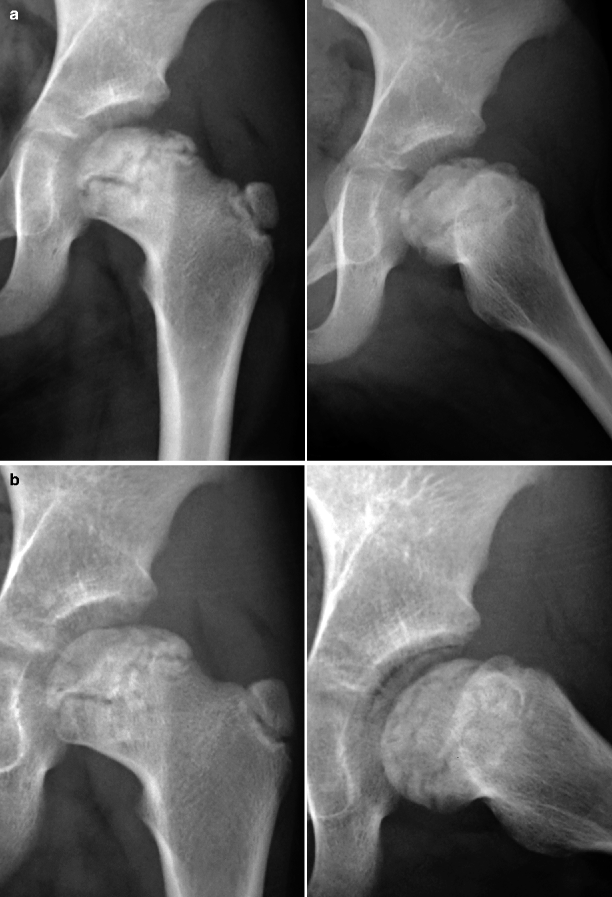
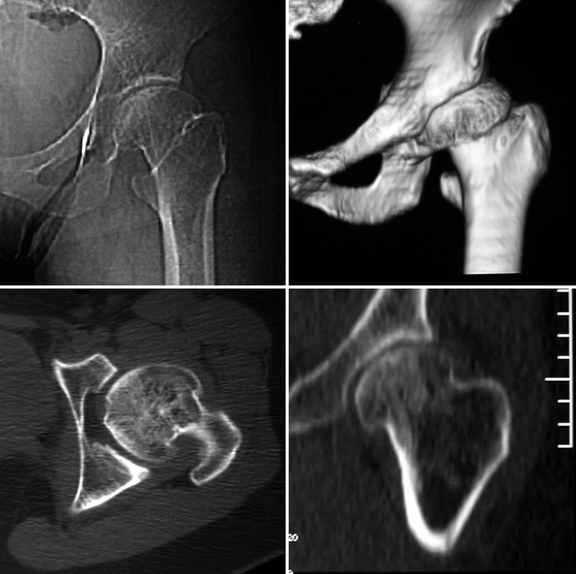
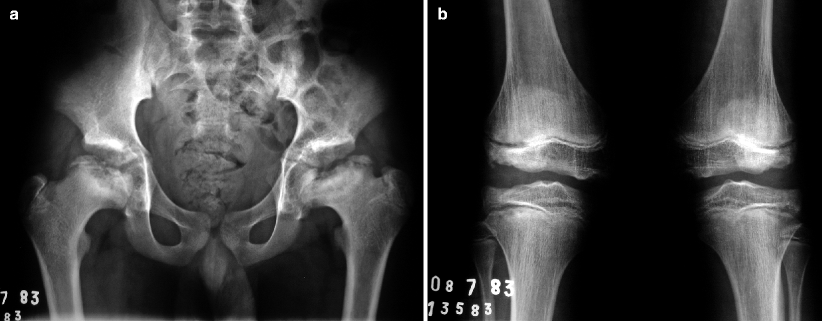

Fig. 7.1
Radiographs of the right hip of a child with LCPD show diffuse sclerosis and reduced height of the femoral epiphysis, as well as a subchondral fracture, more evident on the lateral view (right image). Widening of the joint space is also present

Fig. 7.2
Lateral view of the left hip (a) of a patient with LCPD displaying reduced height of the femoral epiphysis and a crescent-shaped subcortical lucency (subchondral fracture). Transverse CT image (b) of the same patient demonstrates gas in the fracture site and epiphyseal sclerosis

Fig. 7.3
Marked epiphyseal sclerosis is seen in this anteroposterior view of the left hip, with flattening of the articular surface and collapse of the lateral two-thirds of the ossified portion of the femoral head. There is also mild lateral subluxation of this epiphysis

Fig. 7.4
Metaphyseal reaction in the right hip of a patient with LCPD. There are multiple well-delimited, confluent lucencies adjacent to the physis, with broadening of the femoral neck. The femoral head is dense, with focal lucencies permeating the sclerotic bone

Fig. 7.5
The left proximal femoral epiphysis shows decreased density and size when compared with the contralateral one in this radiograph, findings related to LCPD. Metaphyseal reaction is evident, as well as slight widening of the joint space

Fig. 7.6
The left proximal femoral epiphysis is flattened and fragmented due to ischemia, with a centrally located sequestrum. There is remodeling of the femoral neck, which is broadened and shortened, in addition to obvious widening of the joint space and extrusion of the lateral portion of the femoral epiphysis

Fig. 7.7
Radiographs of the hips of a patient with bilateral LCPD displaying fragmentation and reduced size of the left proximal femoral epiphysis, with ipsilateral metaphyseal reaction and broadening of the femoral neck. The right femoral head is also flattened, sclerotic, and irregular

Fig. 7.8
Radiographs of both hips showing flattening and fragmentation of the right proximal femoral epiphysis, affecting mainly its lateral two-thirds, with evident metaphyseal reaction. There is partial extrusion of the right femoral head and remodeling of the acetabular cavity, which is widened and shallow. The right femoral neck is short and broad

Fig. 7.9
In (a), radiographs of the left hip of a child with LCPD display flattening and fragmentation of the proximal femoral epiphysis affecting mainly its lateral third, with a wavy, M-shaped growth plate. The femoral neck is broadened and shortened, with sclerosis of the medial cortex, representing a response to anomalous overload. In (b), radiographs taken 9 months later demonstrate coalescence of bone fragments, with reossification of the lateral pillar and partial restoration of the convexity of the articular surface. Nonetheless, the epiphysis is deformed and partially extruded

Fig. 7.10
Late-stage sequelae of LCPD in a young adult. Radiograph (upper-left image), volume-rendered reconstruction (upper-right image), transverse CT section (lower-left image), and reformatted coronal image (lower-right image) demonstrate morphologic abnormalities of the left femoral head (flattening and loss of its spherical shape, lateral subluxation, and joint incongruity) and femoral neck (shortening and broadening), with secondary osteoarthritis

Fig. 7.11




Radiographs of the pelvis (a) and of the knees (b) of a child with multiple epiphyseal dysplasia. Radiographic findings in the hips resemble those found during the fragmentation phase of LCPD. However, as these findings are bilateral and symmetric and considering that concomitant morphologic epiphyseal abnormalities are also found in the distal femora and proximal tibiae, these features are indicative of their dysplastic nature
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree