Reference, year
Device
Lobar
FUP interval
No. patients
Cirrhosis
Child B
Grade > 3 Bilirubina
Ascites
Findings
(Dancey et al. 2000)
Glass
0 %
nr
22
nr
nr
22 %*
nr
2 potential REILD-related deaths
(Geschwind et al. 2004)
Glass
65 %
Any time
80
nr
10 %
28 %*
7 %
1 treatment-related death
(Carr et al. 2004)
Glass
100 %
0–180
65
75 %
nr
38 %*
12 %
1 definite and 2 probable treatment-related deaths
(Salem et al. 2005)
Glass
51 %
0–90
43
60 %b
nr
14 %
7 %
(Sangro et al. 2006)
Resin
46 %
0–90
24
71 %
0 %
12 %
2 treatment-related deaths
(Kulik et al. 2008)
Glass
nr
0–180
82
100 %
33 %
40 %*
18 %
(Kulik et al. 2008)
Glass
nr
0–180
26
0 %
–
4 %*
4 %
(Hilgard et al. 2010)
Glass
most
0–90
108
76 %
22 %
23 %
0 %
(Salem et al. 2010)
Glass
nr
Any time
291
87 %
55 %c
19 %
nr
(Sangro et al. 2011)
Resin
55 %
0–90
325
78 %
17 %
5 %
nr
(Mazzaferro et al. 2012)
Glass
94 %
0–90
52
100 %
17 %
13 %
8 %
23 % of patients developed liver decompensationd
Table 2
Liver-related adverse events among patients with liver metastases from colorectal carcinoma treated by radioembolization
Ref, year | Device | Lobar | FUP Interval | No. patients | Prior chemo | Concurrent chemo | Grade > 3 bilirubina | Ascites | Findings |
|---|---|---|---|---|---|---|---|---|---|
(Sharma et al. 2007) | Resin | 25 % | Any time | 20 | 1st line | FOLFOX6 | 10 % | – | |
(Van Hazel et al. 2004) | Resin | – | Any time | 11 | 1st line | 5FU/LV (Mayo) | 0 %** | No difference with 5FU/LV alone | |
(Gray et al. 2001) | Resin | – | Any time | 35 | 1st line (86 %) | IA FUDR | 3 %** | RCT nonsignificant difference with FUDR alone | |
(van Hazel et al. 2009) | Resin | nr | Any time | 25 | 2nd line (68 %) | Irinotecan | 4 %** | – | |
(Seidensticker et al. 2011) | Resin | 14 % | Any time | 29 | Salvage | No | – | – | 3 % REILD |
(Hendlisz et al. 2010) | Resin | 0 % | Any time | 21 | Salvage | Protracted Iv 5FU | – | – | RCT No liver-related toxicities |
(Mulcahy et al. 2009) | Glass | 17 % | Any time | 72 | Salvage | No | 13 % | ||
(Jakobs et al. 2008) | Resin | – | Any time | 41 | Salvage | No | – | – | |
(Kennedy et al. 2006) | Resin | 27 % | Any time | 208 | Salvage | No | 1.5 % | – | No VOD |
(Lim et al. 2005) | Resin | – | Any time | 30 | Salvage | 5FUb | nr | nr | 1 RILD |
Table 3
Liver-related adverse events among patients with liver metastases from neuroendocrine tumors treated by radioembolization
Reference, year | Device | Lobar | FUP Interval | No. patients | Prior Treat | Concurrent chemo | Grade > 3 Bilrubina | Ascites | Findings |
|---|---|---|---|---|---|---|---|---|---|
(Ezziddin et al. 2012) | Resin/Glass | 22 % | 0–180 | 23 | PRRT | No | 9 % | – | |
(King et al. 2008) | Resin | – | Any time | 32 | SSA 15 % Chemo | 5FU | nr | – | 1 jaundiced patient |
(Kennedy et al. 2008) | Resin | 59 % | 0–90 | 148 | nr | nr | nr | 0.5 % |
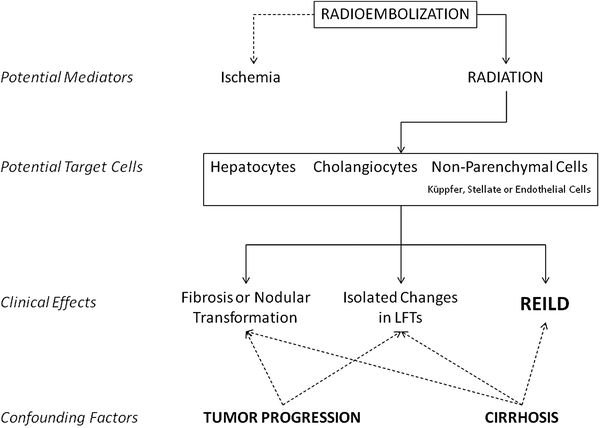
Fig. 1
Potentially relevant factors in the development of liver toxicities after radioembolization of liver tumors
The incidence of REILD in other series cannot be established because they usually report separately those individual parameters such as increased bilirubin or ascites. And they do it along different periods of time, from 30 days to the entire follow-up period. Patients with liver tumors, particularly if they are cirrhotics, may develop hyperbilirubinemia or ascites as a consequence of tumor or cirrhosis progression. A causal relationship between RE and these findings may thus be confirmed only in controlled clinical trials in which adverse events are recorded prospectively and compared to those occurring in a control arm. However, it is very likely that the increased bilirubin levels reflect REILD in a significant fraction of these patients with early hyperbilirubinemia. This opinion is further supported by the fact that the early increase in bilirubin is not associated with other changes that may reflect abating liver function such as decreased albumin levels or prothrombin activity, even in cirrhotic patients (Sangro et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree