9
Liver Malignancies
Jared R. Robbins
INTRODUCTION
Each year in the United States there are estimated to be over 45,000 cases of primary liver and biliary system malignancies with over 28,000 deaths, and the incidence is rising.1 Worldwide primary liver malignancies are the 6th most prevalent site of cancer, but in developing countries it is the 3rd most prevalent cancer.2 Primary liver cancer exacts a significant toll on the worldwide population, causing over 700,000 deaths annually and is the third most common cause of cancer death overall, 2nd in men, and 6th in women.3 While primary cancer is a major global health problem, secondary metastases to the liver are more common. Liver is the primary site of metastases for many malignant neoplasms, particularly those of the gastrointestinal (GI) tract because their draining blood supply is funneled into the portal venous system. Colorectal cancer is the third most common malignancy in the United States, the second leading cause of cancer death, and represents the majority of liver metastases. It is estimated that in 2015 there will be greater than 130,000 new cases of colorectal cancer in the United States.1 Some estimate that 50% to 70% of patients with locally advanced colorectal cancer will eventually develop liver metastases. Aside from cancers of the GI tract (colorectal, stomach, pancreas, and esophagus), liver metastases frequently arise from lung cancer, breast cancer, and melanoma.
Patients with primary malignancy of the liver or liver metastases have relatively poor prognosis. The 5-year survival for primary liver cancers is less than 20%. Untreated hepatocellular carcinoma (HCC) has a median survival of 3 to 8.3 months.4,5 Patients with limited disease that can be surgically treated may have improved long-term survival. The 5-year overall survival rate for individuals with early stage HCC who undergo liver transplants is 44% to 78%, while the 5-year survival for those undergoing liver resection is 27% to 70%.6 Liver metastases also have poor prognoses, with patients with multiple liver metastases having a median survival of only a few months (2.5–7 months), but some patients with limited liver and systemic disease may have much longer survival after local therapies like surgery, liver ablation, or stereotactic body radiation therapy (SBRT).7-12
Although primary liver malignancies or metastases may be asymptomatic for some time after development, eventually untreated or recurrent disease in the liver will result in significant morbidity and symptoms. These can include abdominal pain, feeling of poor health or weakness, loss of appetite, weight loss, nausea/vomiting, fever, fatigue, bloating, abdominal distention, itching, lower extremity edema, jaundice, and liver failure. A summary of the frequency of the various symptoms can be seen in Table 9.1.
The four most common tumor types that require palliative liver treatments are HCC, cholangiocarcinoma, metastatic colorectal carcinoma, and metastatic neuroendocrine tumors.2,15 While each may have slightly different presentations and symptoms secondary to differences in location, natural history, and histology, all have similar options for palliative treatments. Palliative methods for managing symptoms from advanced liver tumors including resection, ablation, vascular intervention, stenting, chemotherapy, medical management, and radiation therapy.15 The proper application of each intervention varies somewhat by both patient-related and tumor-related factors. Appropriate selection of the most useful intervention is outside the scope of this chapter, but when possible, discussion in a multidisciplinary team (surgeons, interventional radiologists, medical oncologists, radiation oncologists, palliative care specialists) can help facilitate the most appropriate action for each patient. The purpose of this chapter is to discuss the use of external beam radiation therapy for palliation of liver malignancy and will focus primarily on external beam radiation therapy, but will also briefly discuss the use of SBRT and radioembolization.
In the early days of external beam radiation therapy with both cobalt units and newly minted linear accelerators, various groups started using whole liver external beam techniques (WLRT) to palliate symptomatic liver metastases. In the 1950s to 1970s, several groups published their retrospective institutional experiences.9,10,16-19 These initial pioneering efforts showed great promise with effective palliation in the majority of patients with a reasonable toxicity profiles, with WLRT doses ranging from around 19 Gy to 31 Gy delivered in 2.5 to 4 weeks courses.9,10,16-19 They also realized the sensitivity of the liver to whole liver radiation doses of more than 30 Gy, which could result in radiation-induced hepatitis or radiation-induced liver disease (RILD).17
TABLE 9.1 Frequency of Symptoms Associated With Advanced or Refractory Primary and Secondary Liver Malignancies

Building on the enthusiasm of these early experiences, the first multi-institutional trial evaluating the use of WLRT for the palliation of malignant liver disease was performed by the Radiation Therapy Oncology Group (RTOG 76-05). Incidentally, this was also the first RTOG study of a GI site, perhaps emphasizing the burden of symptomatic liver metastases at that time. This was a prospective, uncontrolled, nonrandomized pilot study designed to test the feasibility of hepatic irradiation for symptomatic metastatic liver disease using six separate radiation schemas (two for solitary metastases and four for multiple hepatic lesions or patients with metastatic disease outside of the liver). The regimens for solitary metastases included the initial whole liver fields followed by an optional boost to the solitary lesion up to 1/3 of the liver, while patients with multiple hepatic metastases were treating to the whole liver only. Specific details about the radiation therapy (RT) can be found in Table 9.2. Seventy-seven percent of the patients completed the treatment, while seven died prior to the completion of the RT. The median survival was 11 weeks. The most common side effect from the RT was nausea/vomiting in 16%. No patient developed radiation hepatitis, nephritis, or pneumonitis. Eight specific signs and symptoms were evaluated at 4 weeks post-treatment, and improvements were seen in all symptoms with the best responses for abdominal pain (55%), nausea/vomiting (49%), and fever/night sweats (45%). Complete responses for symptoms ranged from 7% (weakness/fatigue) to 34% (nausea/vomiting). Response rates were highest for those with the most symptomatic disease. Karnofsky performance status improved in 25% of patients and liver function improved in 37% to 48% of patients who presented with abnormal values. Overall, the authors estimated that patients with mild or no symptoms spent an average of 80% of their remaining lives with mild to no pain, while those with moderate or severe pain before treatment spent an average of 63% of their remaining lives with either mild or no pain.8 Overall, this study was viewed as a success and was a foundation for further inquiry.
During the subsequent decade after the closure of RTOG 76-05, the RTOG initiated six additional trials for primary or metastatic liver malignancy. Five of the six trials used WLRT with various combinations of radiosensitizers, chemotherapies, radiopharmaceuticals, or alterations in the radiation delivery in attempts to improve the therapeutic benefit of RT. These strategies were pursued because radiation dose was limited by the radiosensitivity of the normal liver.11,20-24 The standard radiation regimen for these studies was 21 Gy in 7 fractions.20-22,24 In RTOG 80-03, patients were randomized to this regimen alone or in combination with misonidazole, a compound that selectively sensitizes hypoxic tumor cells to radiation. Although the addition of a radiosensitizer didn’t improve the therapeutic response in any of the tested parameters, the study confirmed the benefits of WLRT. In this cohort, 80% had their abdominal pain effectively palliated with 54% of these achieving complete remission. Palliation was relatively rapid with median time to relief of 1.7 weeks with a median duration of 13 weeks. For patients who lived 3 months, 52% maintained their improved pain status. Follow-up CT scans evaluated tumor response. Although the response was not robust by imaging criteria (0.6% complete response, 7% partial response, and 13% marginal response), patients still experienced symptomatic relief with the treatment.21
TABLE 9.2 Select Prospective Trials of Palliative RT to Liver




Investigations of chemotherapy and radiopharmaceuticals in addition to WLRT for primary liver tumors (RTOG 79-28, RTOG 83-01, RTOG 83-19), showed improved radiographic response by CT radiography compared to historical values (48% response rate with 7% complete and 41% partial), but there was increased toxicity and no survival benefit.20,23-25
An accelerated hyperfractionated study (RTOG 84-05) sought to determine if higher radiation doses could prolong survival and to gain a better understanding of liver tolerance to fractionated external beam radiation therapy. In this study, 173 patients with liver metastases from a primary GI site received 1.5 Gy twice a day to the whole liver with sequential groups receiving 27 Gy, 30 Gy, and 33 Gy total doses. This mild dose escalation did not prolong survival or decrease the rate of death from liver metastases, but five of the 51 patients entered on the 33 Gy group experienced late liver injury with an actuarial risk of severe radiation hepatitis (grade 3 or higher) of 10% at 6 months. Since no liver toxicity was observed in the 27 Gy and 30 Gy groups, this was thought to be a safe regimen.11
In addition to the previous studies using 21 Gy in 7 fractions as the standard dose for WLRT, two additional prospective trials investigated the effectiveness and tolerability of shorter radiation courses.13,14 The first was a 28-patient cohort with symptomatic liver metastases treated with partial or whole liver irradiation of 10 Gy in 2 fractions given over 2 days. Individual symptom response rate for pain score, abdominal distention, night sweats, nausea, and vomiting were 53% to 66% at 2 weeks with partial or complete global symptomatic responses in 54% of treated patients. The majority (75%) of patients perceived benefit from the treatment. Toxicity was relatively limited with 7% experiencing grade 3 toxicity within 2 weeks of therapy (one episode of nausea, one episode of diarrhea) and 14% did have transient temporary increase in pain shortly after treatment.13 The second study included 41 symptomatic patients with either primary or secondary liver malignancy who received 8 Gy to the whole liver in one fraction. One particular innovation of this study was the prospective utilization of the validated patient-reported symptoms and quality of life metrics [Brief Pain Index (BPI)], Functional Assessment of Cancer Therapy—Hepatobiliary (FACT-Hep), and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30)). At one month, 48% had an improvement in symptoms. Improvements in the FACT-G and hepatobiliary subscale were seen in 23% to 29% of patients at 1 month. Improvements in EORTC QLQ-C30 functional (range 11%–21%) and symptom (range 11%–50 %) domains were also observed. Grade 3 toxicity was limited to one patient who declined premedication and developed nausea.14 While these studies suggest useful palliation from short courses of whole liver irradiation, there are currently no published studies directly comparing any of the palliative whole liver radiation therapy regimens in a prospective randomized fashion. There is an ongoing phase III randomized trial by the National Cancer Institute of Canada (NCIC) HE1, of best supportive care compared to palliative radiation therapy for patients with HCC or liver metastases.
When managing patients with malignant liver disease with radiation therapy, the dose-limiting toxicity is usually RILD.17,22,26 This has led to the development of low-dose WLRT as has been described. While this therapy can offer good palliation, the durability of local control, symptom palliation, and radiographic response may be limited. With the development of three-dimensional (3D) conformal radiation therapy, improvements in radiation therapy delivery techniques, and enhanced understanding of the partial tolerance of the liver to radiation therapy, ablative doses of radiation can safely be delivered to patients with limited liver disease with a potential for improved local control and durability of response. Initial efforts using 3D conformal radiation showed that even with high doses of radiation, the risk of RILD was low as long as the mean liver dose was in the range shown to be safe from the WLRT experiences (~30 Gy).27 Several predictive models have been developed, which help estimate the risk of RILD.28-30 These models use parameters like the whole liver dose associated with a 50% probability of toxicity; the volume of the liver receiving radiation, and even the type of malignancy to help predict if a given radiation plan is safe to deliver.
Greater understanding of the principles surrounding the partial volume tolerance of the liver to radiation coupled with improved visualization of liver tumors and more precise radiation therapy delivery techniques has led to the development of SBRT for liver tumors. This technique, which was initially developed for treating early stage lung cancer, uses increased conformity and steep dose gradients to deliver hypofractionated ablative doses to the target volume within the liver, while keeping the mean liver dose low and avoiding nearby critical structures. In comparison to WLRT, liver SBRT requires more complex treatment planning, more awareness, understanding, and management of organ motion (during and between treatments), more dependence on image guidance, more rigorous quality assurance and coordination, and increased cost, but has the potential for greater tumor control and improved treatment durability.31 While WLRT is typically used in symptomatic patients with potentially widespread or multifocal liver and systemic disease, limited performance status, and relatively short survival potential, optimal patients for SBRT have limited liver and systemic disease, reasonable performance status, a potential for longer survival, and are typically asymptomatic from their liver malignancy. Several prospective trials have been conducted for both metastatic and primary liver lesions.7,32 Overall, for liver metastases the 1-year local control ranges from 71% to 95% with limited reports of toxicity, and median survival rates of 17 to 20 months.12,33-35 Likewise, prospective trials of SBRT for hepatocellular carcinoma yield similar high 1-year local control (75%–100%), limited toxicity, and reasonable 1-year overall survival rates of 55% to 75%.36-39 Recent evidence also supports that SBRT causes no detriment to patient reported quality of life.40
One additional clinical situation where liver 3D conformal RT or SBRT may play an important role in palliation is in the case of HCC with portal vein thrombosis (PVT). PVT carries a poor prognosis with survival of only a few months without treatment. Many patients with PVT may not be eligible for standard therapies like surgery or radiofrequency ablation. There are multiple retrospective as well as several prospective studies evaluating 3D RT and SBRT in patients with PVT.36,41-44 These studies suggest that radiation may improve vein patency and improve survival. The largest prospective study is from Princess Margaret Hospital, where they treated 56 patients with portal vein tumor thrombus with a 30 Gy to 54 Gy in 6 fractions regimen (median dose was 36 Gy in 6 fractions). They reported a 1-year overall survival rate of 44% with a median survival of almost 11 months.36 Additional strategies for patients with PVT, for example, combining transarterial chemotherapy embolation (TACE) with radiation have shown promising 1- and 2-year overall survival rates of 42.5% and 22.8%.44 Likewise a study investigating the addition of RT to percutaneous transhepatic portal vein stenting and TACE showed improved 1-year survival (32% vs. 6.9%, p < 0.01) for the patients receiving the additional RT.43 Together, these data support the consideration of RT for patients with HCC and PVT.
Biliary obstruction caused from external compression of the bile duct or from tumor growing in the duct can be a significant cause of morbidity in patients with both primary and secondary liver malignancies. While biliary obstruction can be managed emergently through percutaneous drainage followed by conversion to internal stent or internal stent upfront in less emergent cases, external beam radiation may be helpful in select patients. Although the data is limited, some evidence suggests a benefit for adding radiation as a palliative measure to help keep stents patent longer or to help open externally compressed biliary ducts.45-47
In addition to the various forms of external beam radiation therapy, there is growing interest in radioembolization. This minimally invasive outpatient technique uses interventional radiological procedures to isolate the arterial supply of a liver tumor and selectively delivers radioactive material into the tumor vasculature. Yttrium-90 (Y-90) is the most commonly used isotope and can be attached to glass or resin beads. It is a pure-beta emitter with a half-life of about 64 hours and a tissue penetration range of 2.5 mm to 11 mm. With this technique, single or multiple tumors can be treated. Clinically, Y90 has been shown to slow progression and control both primary and secondary liver cancers.48,49
PLANNING
Simulation
Patients should be simulated supine with a CT scan with adequate immobilization to ensure reproducible set up with their arms above their heads. Estimates of respiratory motion should be acquired at time of simulation with either a 4D respiratory-correlated scan or inhalation/exhalation scans to get an approximation for organ movement. The degree of certainty regarding tumor and organ motion and rigidity for immobilization should reflect the intended radiation plan and technique, with patients undergoing SBRT or high-dose conformal treatments requiring more certainty than those being treated with low-dose whole liver treatments. Similarly, patients receiving more complex treatments may benefit from an MRI simulation, IV/oral contrast, or fiducial placement as part of the treatment planning process. Flouroscopic simulation may also be appropriate for WLRT to determine the degree of excursion of the liver with respiration, as it should be considered when designing treatment fields.
Target Delineation
Whole liver
For whole liver treatments, the gross tumor volume (GTV) should be defined as the area of the liver causing the symptoms. The clinical target volume (CTV) will be the entire liver with the planning target volume (PTV) representing a 1 cm to 2 cm margin around the CTV depending on the set up and immobilization of each patient. Organs at risk (OAR) (stomach, spinal cord, small and large bowel, and kidneys) should also be delineated on the simulation CT scan.
SBRT/high-dose 3D conformal
The GTV is defined by the tumor either on the planning CT or another fused diagnostic study (CT or MRI). For patients with significant motion of the GTV with respiration, a gated technique, active breathing control (ABC), or additional motion control (abdominal compression) is appropriate. For patients treated with a gated technique, the GTV should be delineated for only the phases of respiration when the beam will be on to create an internal tumor volume (ITV). For tumors with less respiratory movement due to position, abdominal compression, or active breath hold, the GTV should be delineated in the same manner as described, but the ITV should be created using all phases of the respiratory cycle. Patient-specific PTV margins ≥5 mm should be added to the ITV depending on patient and treatment factors including tumor respiratory motion, immobilization, and motion management strategy.
Treatment Plan
Whole liver
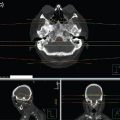
Three-dimensional planning should be used in order to understand the anatomy and guide field design and beam arrangements. Figure 9.1 shows a 3D WLRT plan. Typically, anterior-posterior opposed or oblique plans can be used based on the patient’s anatomy and habitus to provide the best coverage of the whole liver while sparing radiation dose to the OAR. Megavoltage energy beams should be used. To avoid excessive dose to the kidneys, posterior blocks can be used to block the kidneys or they can be placed outside of the fields when possible. Excessively complicated plans should be avoided in severely symptomatic patients to avoid prolonged time on the treatment table. For tumor coverage, the treatment goal of 95% of the PTV receiving a minimum of at least 90% of the prescribed dose should be adequate. The constraints for OARs are dependent on the dose to the whole liver and the fractionation. For 21 Gy in 3 Gy daily fractions, at least two-thirds of one kidney should be shielded or outside of the radiation field.21 For 10 Gy in 5 Gy fractions, shielding of the kidney is recommended when possible and should be required for one kidney if both would be in the radiation field.13 For 8 Gy in a single fraction, the maximum dose to the stomach, small bowel, and spinal cord must be less than 10 Gy.14

FIGURE 9.1 Radiation plan for whole liver external beam radiation therapy for a 65-year-old male with diffuse hepatic metastases causing abdominal pain. (a, b) Plan AP with a slight posterior oblique to partially avoid the right kidney. (c–e) Isodose lines and dose-volume histogram show coverage and the dose to surrounding organs.
SBRT/High-dose 3D
3D planning should be used to determine beam arrangement and the most appropriate technique. Figure 9.2 shows a typical SBRT plan for a single liver metastasis. Techniques such as dynamic conformal arcs or multiple planar or noncoplanar beams (dynamic or static) with either 3D conformal or intensity modulated radiation therapy can be used. Beam energies should range from 6 to 18 MV. Efforts should be made to make treatment conformal to avoid excessive dose to the liver or surrounding structures. Constraints to OARs will vary slightly depending on the prescription dose and the number of fractions. Refer to Table 9.3 for suggested constraints.
Day of Treatment
Premedication
All patients should receive premedication with an oral antiemetic (ondansetron) and low dose dexamethasone about one hour prior to treatment. Patients with a substantial dose to the stomach or bowel may also benefit from an antacid to help reduce the risk of ulcer.

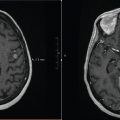
FIGURE 9.2 (a) SBRT plan for a 73-year-old male with a single 4.3 cm focus of metastatic melanoma to the liver. Treated with 50 Gy in 5 fractions. (b) Post-SBRT imaging at 3 months shows excellent tumor response with no observed acute toxicity.
TABLE 9.3 General Constraints for Liver SBRT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree