Presentation
Resectability
Recommended treatment
T1-2N0
Operable
Lobectomy (preferred over segmentectomy or wedge resection) or SBRT
Inoperable
SBRT (may consider RFA/Cryotherapy)
II (T2bN0, T1-2N1, T3N0)
Operable
Surgery → chemo (>4 cm)
Inoperable
ChemoRT → ±chemo or hypofx EBRT → ±chemo
IIIA
Operable
ChemoRT → restage → surgery → chemo or Chemo → restage → surgery → chemo ± RT
Inoperable
ChemoRT → ±chemo
IIIB
Inoperable
ChemoRT → ±chemo
Recurrent
Operable
EBRT/SBRT/resection for limited local recurrence → systemic therapy
Inoperable
EBRT/SBRT/RFA/cryo for limited recurrence → systemic therapy
Pulmonary oligometastases
Operable
Lobectomy/wedge resection or SBRT or hypofractionated EBRT (for larger lesions, >5 cm) → systemic therapy
Inoperable
SBRT, RFA, cryo, or hypofx EBRT (preferred for larger lesions, > 5 cm) → systemic therapy
Radiosurgical Technique
Simulation and Treatment Planning
Tumor motion may be 2–3 cm in peri-diaphragmatic regions of the lower lung. Motion management strategies include respiratory gating , coaching with audio-visual feedback, breath-hold techniques, abdominal compression , and intrafraction tumor tracking real-time imaging techniques with dynamic beam and/or couch compensation.
Thin-cut CT (≤1.5 mm) thickness recommended. 4DCT or maximal inspiratory and expiratory phase CTs or slow CT recommended to assess target and critical structure internal motion. Free-breathing helical or mean intensity projection CT should be used for dose calculation.
iGTV contoured from Maximum Intensity Projection (MIP) generated from 4DCT. MIP should be used judiciously in tumors adjacent to diaphragm or chest wall , with additional imaging as needed to fully discriminate the target from surrounding normal tissue with similar CT tissue density.
GTV /iGTV = tumor visible on CT lung window.
CTV /ITV = GTV /iGTV + 0–10 mm (in RTOG protocols , GTV and CTV have been considered identical on CT planning with zero expansion margin added).
PTV = CTV /ITV + 3–10 mm (dependent upon available center-specific IGRT and motion management capabilities). Current RTOG guidelines are:
Non-4DCT planning, PTV = GTV + 5 mm axial and 10 mm longitudinal anisotropic margins.
4DCT planning, PTV = ITV + 5 mm isotropic margin.
Dose to proximal OARs attributed to compact intermediate dose region outside of the CTV /ITV region, generally reduced with increased beams and angles, as well as minimization of margins on target.
Treatment planning guidelines (adapted from RTOG 0618).
VRx dose ≥95 % PTV , V90 ≥99 % PTV.
High dose region (≥105 % Rx dose) should fall within the PTV .
Conformality Index goal ≤1.2.
Heterogeneity correction algorithms are increasingly routinely used for planning (anisotropic analytical algorithm, collapsed cone convolution, Monte Carlo , etc.). Pencil-beam algorithms that overestimate dose in heterogeneous tissue are generally not recommended.
Phantom-based QA on treatment plans.
Dose Prescription
Dose and fractionation directed by adjacent normal tissue RT toxicity constraints with goal tumor BED10 > 100. Adaptive dosimetry for histology -, volume-, location-, and context-based lesions (primary vs. metastatic) are under investigation.
Current dose fractionation schema largely employs 1–5 fractions.
Peripheral Lung Tumors
Common accepted schemas: 25–34 Gy × 1 fraction, 18 Gy × 3 fractions, 12 Gy × 4 fractions, 10 Gy × 5 fractions.
Central Lung Tumors
We recommend: 10 Gy × 5 fractions (BED10 dose limited to reduce toxicity of central structures: large airways, heart , esophagus , and spinal cord ). See Fig. 7.1.
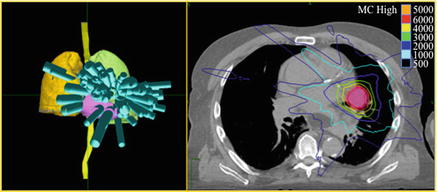
Fig. 7.1.
SBRT planning for a central early-stage NSCLC. Beam distribution shown on 3D anatomy reconstruction (left) and dose distribution for 50 Gy given in 5 fractions (right)
Dose typically prescribed 60–90 % IDL , with ≥95 % PTV coverage by prescription dose.
Composite planning should be employed in cases of regional lung re-irradiation with appropriate BED conversion for dose summation.
Dose Limitations
See Table 7.2, assuming no prior regional radiotherapy (TG101, Benedict et al., 2010; RTOG 0618).
Table 7.2
Recommended dose constraints for SBRT lung lesion target planning
Structure
Fractions
Constraints
Lung
1
V7 < 1500 cc
3
V11.6 < 1500 cc
5
V12.5 < 1500 cc
Central airway
1
V10.5 < 4 cc, Dmax 20.2 Gy
3
V15 < 4 cc, Dmax 30 Gy
5
V16.5 < 4 cc, Dmax 40 Gy
Chest wall
1
V22 < 1 cc, Dmax 30 Gy
3
V28.8 < 1 cc, Dmax 36.9 Gy
5
V35 < 1 cc, Dmax 43 Gy
Heart
1
V16 < 15 cc, Dmax 22 Gy
3
V24 < 15 cc, Dmax 30 Gy
5
V32 < 15 cc, Dmax 38 Gy
Esophagus
1
V11.9 < 5 cc, Dmax 15.4 Gy
3
V17.7 < 5 cc, Dmax 25.2 Gy
5
V19.5 < 5 cc, Dmax 35 Gy
Brachial plexus
1
V14 < 3 cc, Dmax 17.5 Gy
3
V20.4 < 3 cc, Dmax 24 Gy
5
V 27 < 3 cc, Dmax 30.5 Gy
Spinal cord
1
V10 < 0.35 cc, Dmax 14 Gy
3
V18 < 0.35 cc, Dmax 21.9 Gy
5
V23 < 0.35 cc, Dmax 30 Gy
Skin
1
V23 < 10 cc, Dmax 26 Gy
3
V30 < 10 cc, Dmax 33 Gy
5
V36.5 < 10 cc, Dmax 39.5 Gy
Dose Delivery
Dose delivered in consecutive daily or every other day fractions as per current NRG protocols.
Setup may be isocentric or non-isocentric depending upon SBRT delivery system.
Verification by kV XR or CBCT , aligned to visualized tumor or surrogate.
Intrafraction dose delivery adjustment by motion management and IGRT systems as discussed above.
Toxicities and Management
Common acute toxicities (<6 weeks):
Fatigue
Generally early-onset and self-limiting.
Sustained fatigue may be related to cardiopulmonary dysfunction (CHF, CAD, COPD, etc.) and warrants further work up.
Cough/dyspnea
Low-grade cough common secondary to RT-related intrapulmonary inflammation. Antitussive pharmacotherapy for mild symptoms.
Severity of shortness of breath may be related to baseline lung function and associated comorbidities . For patients with moderate to severe symptoms or significant baseline comorbidities (COPD, ILD, CHF, etc.), recommend follow-up with pulmonology and/or cardiology.
Chest pain
May be related to regional pleuritis and/or pericarditis and is generally self-limited.
Analgesic pharmacotherapy recommended.
Pneumonitis
Associated with increased dose volume (V20 <10 %), smoking history (current/former), age, prior use of steroids , and comorbidity index on multiple studies.
Generally subacute onset (>2 weeks), associated with cough , dyspnea , hypoxia , and fever .
If symptomatic, treat with prednisone (1 mg/kg/d) or 60 mg/d and trimethoprim/sulfamethoxazole for PCP prophylaxis. Symptomatic relief may be rapid but slow steroid taper is critical for durable symptom resolution.
Esophagitis
Increased risk with treatment centrally located tumors, and is generally self-limited to several weeks after treatment.
Local or systemic analgesic pharmacotherapy (lidocaine , NSAIDs, opioids) ± proton pump inhibitor based on severity of symptoms.
Dermatitis
Chest wall entrance and exit doses can be reduced with increased numbers of beams to minimize radiation dermatitis.
Mild to moderate skin reaction treated with supportive care , including topical moisturizer s , analgesics , low-dose steroids , and antimicrobial salves.
Common late toxicities (>6 weeks):
Persistent cough /dyspnea
Recommend consultation with pulmonary medicine for consideration of long-term bronchodilator and anti-inflammatory therapy.
Radiation pneumonitis
Most commonly observed at ~6 weeks.
As above, recommend steroids with gradual taper for symptomatic patients
Brachial plexopathy
Apical lung tumors associated with greater risk of brachial plexus injury.
May present with neuropathic pain as seen in Lhermittes syndrome or with motor/sensory changes in the upper extremities.
MRI of brachialplexus and upper spine may be diagnostic and rule out tumor recurrence.
Limited treatment options include supportive care and occupational therapy .
Chest wall pain and rib fracture
More common in patients with peripheral lesions.
Supportive care indicated.
Radiation skin ulcer
For persistent non-healing skin lesions, consider hyperbaric oxygen therapy and tocopherol pharmacotherapy .
Esophageal stricture and tracheoesophageal fistula
Historically rare complication observed with treatment of mediastinal lymphadenopathy in locally advanced lung cancer .
Even less likely with SBRT , if airway and esophageal constraints maintained, with exception of re-irradiation setting.
Vasculopathy
Vascular erosion may lead to limited hemoptysis or massive hemorrhage and death (seen in re-irradiation setting of central lesions).
Recommended Follow-Up
CT or PETCT every 3–4 months × 3 years, every 6 months × 2 years, every 12 months thereafter for routine follow-up.
Assessment with RECIST criteria of limited utility due to wide spectrum of evolving radiographic features following SBRT including diffuse and patchy GGO , consolidation, and/or fibrosis .
In general, radiographic changes include early inflammatory response (≤3 months) followed by resolution of FDG activity and late fibrosis (>6 months) in area of treated lesion which is often dynamic and may evolve over several years.
Persistent increase in size and density of treated tumor on interval CTs in the early post-treatment setting (<12 months) or new densities at later times (>12 months) should be considered suspicious for recurrence, with recommendation for increased frequency of CT, interval PET scan, and consideration of biopsy and/or surgical or radiotherapy salvage procedure.
Role of molecular imaging and circulating tumor markers is under investigation.
Evidence
Primary Lung Cancer
Evidence widely supports efficacy and safety of SBRT in early-stage NSCLC, with optimal patient selection criteria and dose schema emerging as studies mature.
CALGB 39904 (Bogart et al. 2010). Phase I dose-escalation study of 39 stage I (≤4 cm) NSCLC patients. 70 Gy in 29 decreased to 17 fractions. 92.3 % actuarial control, 82.1 % actuarial distant control. No late grade 3 or 4 toxicity.
Onishi et al. (2004). Initial report of retrospective Japanese multi-institutional series of 245 patients with stage I NSCLC treated with SBRT 18–75 Gy in 1–22 fractions with a median follow-up of 24 months. Grade ≥3 toxicity 2.4 %. LR at 3 years for BED ≥ 100 vs. BED < 100 was 8.1 % vs. 26.4 %, p < 0.05 and OS was 88.4 % vs. 69.4 %, p < 0.05, establishing BED ≥ 100 as prescribing criterion.
Nordic Study Group (Baumann et al. 2009). Phase II study of SBRT in 57 patients with medically inoperable early-stage peripheral tumors (40 stage IA, 17 stage IB), treated with 45–66 Gy in 3 fractions. Estimated 3 year LC and OS were 88.4 % and 59.5 %, respectively. Distant metastatic rate 16 %. Risk of any failure increased in T2 vs. T1 tumors (41 % vs. 18 %, p = 0.027).
RTOG 0236 (Timmerman et al. 2010, Stanic et al. 2014). Phase II multicenter trial of 55 patients with medically inoperable early-stage (<5 cm) peripheral NSCLC (44 stage IA, 11 stage IB), treated with 54 Gy in 3 fractions SBRT . Three year primary tumor and involved lobe control was 98 %. Rate of distant failure 22 % at 3 years. OS 56 % at 3 years. Grade 3 and 4 toxicities were 12.7 % and 3.5 %, respectively. Poor baseline PFT not predictive of SBRT-related toxicity.
Timmerman et al. (2006), Farikis et al. (2009). Phase II study of SBRT at Indiana University for T1-2N0 medically inoperable NSCLC patients (n = 70), 60–66 Gy in 3 fractions. LC and OS at 3 years were 88.1 % and 42.7 %, respectively. Grade ≥3 toxicity rates of 10.4 % peripheral vs. 27.3 % central over a median follow-up of 50.2 months (p = 0.088).
JCOG 0403 (Nagata et al. 2012). Phase II trial of SBRT in early-stage NSCLC, stratified by medically operable/inoperable. In medically inoperable arm, 100 patients with stage IA disease received 48 Gy in 4 fractions. LC and OS at 3 years were 88 % and 59.9 %, respectively. For 64 medically operable patients, LC and OS at 3 years were 86.0 % and 76.0 %, respectively. Grade 3 pneumonitis 7 %, overall grade 4 toxicity 2 %.
RTOG 0618 (Timmerman et al. 2013). Phase II trial of 33 patients with medically operable early-stage peripheral NSCLC (<5 cm), treated with 60 Gy in 3 fractions. Completed accrual in 2010 with results presented at ASCO 2013 showing estimated 2 years primary tumor failure rate of 7.8 %, with a median follow-up of 25 months. Local failure , including ipsilateral lobe, was 19.2 %. PFS and OS at 2 years were estimated at 65.4 % and 84.4 %. Grade 3 toxicity was 16 %.
RTOG 0813. Phase I/II dose-escalation trial of medically inoperable centrally located (<2 cm of proximal bronchial tree) early-stage NSCLC (<5 cm). At the time of accrual completion, dose escalated to 60 Gy in 5 fractions. Closed to accrual at 120 patients in 2013. Results are pending.
RTOG 0915 (Videtic et al. 2013). Phase II randomized trial of 34 Gy in 1 fraction vs. 48 Gy in 4 fractions for medially inoperable early-stage peripheral NSCLC (<5 cm). Study completed accrual in 2011 with 94 patients. At 1 year, LC 97.1 % vs. 97.6 %; OS 85.4 % vs. 91.1 %, and PFS 78.0 % vs. 84.4 %. Adverse events were 9.8 % vs. 13.3 %. Based on the favorable toxicity, the 34 Gy in 1 fraction arm will be compared to the 54 Gy in 3 fractions arm of RTOG 0236 in a phase III setting.
Hoppe et al. (2008). Study of 50 stage I NSCLC patients treated with SBRT 60 Gy in 3 fractions or 44–48 Gy in 4 fractions with a median follow-up of 6 months. Skin toxicity was 38 % grade 1, 8 % grade 2, 4 % grade 3, and 2 % grade 4. Reduced number of beams, proximal distance to chest wall , and skin dose ≥50 % prescription dose were associated with increased risk of skin toxicity.
Mutter et al. (2012). Retrospective study of 128 early-stage NSCLC patients receiving SBRT 40–60 Gy in 3–5 fractions. With a median follow-up of 16 months, grade ≥2 chest wall toxicity was 39 % estimated at 2 years. On dosimetric analysis, grade ≥2 chest wall pain was associated with a V30Gy >70 cm3 within a 2 cm 2D-ipsilateral chest wall expansion.
ACOSOG Z4099/RTOG 1021. Phase III trial of SBRT vs. sublobar resection for high-risk operable, early-stage peripheral NSCLC (<3 cm). Terminated for poor accrual.
ROSEL Trial (VUMC, NCT00687986). Phase III trial of SBRT (60 Gy in 3 or 5 fractions) vs. surgery for stage IA peripheral NSCLC. Terminated for poor accrual.
STARS Trial (MDACC, NCT00840749). Phase III trial of SBRT 60 Gy in 3–4 fractions based on lesion location vs. surgery for stage I NSCLC. Terminated for poor accrual.
Grills et al. (2010). Retrospective comparison of 124 patients (95 % medically inoperable) T1-2 N0 NSCLC receiving wedge resection (n = 69) vs. SBRT (n = 58), 48–60 Gy in 4–5 fractions. No statistically significant differences in LRR (27 % wedge vs. 9 % SBRT, p > 0.16) or CSS (94 % wedge vs. 93 % SBRT, p = 0.53) noted at a median follow-up of 30 months. OS favored wedge resection (87 % wedge vs. 72 % SBRT, p = 0.01).
Crabtree et al. (2010). Retrospective comparison of stage I NSCLC patients receiving either surgery (n = 462) or SBRT (n = 76) for definitive care. Surgical candidates had fewer medical comorbidities . Thirty-five percent of surgical patients were upstaged on final pathology. OS 5 years 55 % surgery and OS 3 years 32 % with SBRT. In propensity matched analysis, no statistically significant difference in LC (88 % vs. 90 %) and OS (54 % vs. 38 %) at 3 years in surgery vs. SBRT groups.
SEER -Medicare analysis (Shirvani et al. 2012). Comparative outcomes of stage I NSCLC patients ≥60 years old, which demonstrated overall ranked outcomes as lobectomy > sublobar resection > SBRT > conventional EBRT > observation. However, as treatment outcomes were likely influenced by patient selection and comorbidities , there was no difference in OS between SBRT and surgical modalities on propensity matched analysis, and EBRT remained inferior to SBRT.
Shah et al. (2013a, b). Cost-effectiveness comparison of surgical resection vs. SBRT for stage I NSCLC patients >65 years. For marginally operable patients, SBRT was most cost effective with a mean cost and quality-adjusted life expectancy of $42 k/8.0 years vs. lobectomy at $49 k/8.9 years. However, for completely operable candidates, lobectomy was found more cost effective, having an incremental cost-effectiveness ratio of $13 K/quality-adjusted life year .
Table 7.3 summarizes several multiple primarily retrospective series indicating local control rate s of 85–95 % at 3–5 years, and overall survival rates of 50–95 % at 3–5 years for early-stage NSCLC managed with SBRT . Some series include limited numbers of recurrent and metastatic patients.
Table 7.3
Selected studies of SBRT treated NSCLC patients
Study
Patients
Treatment
LC/OS
Notes
Onishi et al. JRS-SBRTSG (IJROBP 2013)
2226 patients stage I NSCLC
32–70 Gy in 3–12 fractions, median BED 107 Gy (range 58–150 Gy)
3 years LC/OS 85 %/72 %
3 years LPFS 87 % T1, 72 % T2
3 years OS 75 % BED ≥ 100 Gy vs. 63 % BED < 100 Gy (p < 0.01)
2.9 % grade ≥3
Grills et al. Multi-institutional (JTO 2012)
482 patients (505 tumors) T1-3N0 NSCLC,
87 % medically inoperable
20–64 Gy in 1–15 fractions, median 54 Gy in 3 fractions
2 years LC/OS 94 %/60 %,
LC 96 % BED ≥ 105 vs. 85 % BED < 105 (p < 0.001)
7 % grade ≥2 pneumonitis
3 % rib fracture
Shibamoto et al. Japan (IJROBP 2013, Cancer 2011)
180 patients stage I NSCLC (120 medically inoperable, 60 operable)
Volume-adapted
44 Gy in 4 fractions <1.5 cm, 48 Gy in 4 fractions 1.5–3.0 cm, 52 Gy in 4 fractions >3.0 cm
3 years LC/OS
83 %/69 %, OS 74 % operable vs. 59 % inoperable, LC 86 % ≤3 cm vs. 73 % >3 cm
5 years LC/OS
82 %/68 %
13 % grade ≥2 pneumonitis
Uematsu et al. Japan (IJROBP 2001)
50 patients T1-T2N0 NSCLC (21 medically inoperable, 29 operable)
50–60 Gy in 5–10 fractions (18 patients received 40–60 Gy in 20–33 fractions prior to SBRT)
3 years LC/OS
94 %/66 %
(86 % OS in medically operable subgroup)
4 % rib fracture
Stephans et al. Cleveland Clinic (JTO 2009)
94 patients stage I NSCLC medically inoperable
50 Gy in 5 fractions,
60 Gy in 3 fractions
1 year LC/OS
97 %/83 % 50 Gy in 5 fractions, 100 %/77 % 60 Gy in 3 fractions
2.2 % grade 2 pneumonitis
10 % grade 1–2 chest wall toxicity
Olsen et al. Wash U. (IJRBOP 2011)
130 patients early-stage NSCLC
Peripheral tumors 54 Gy in 3 fractions, central tumors 45–50 Gy in 5 fractions
2 years LC
91 % (54 Gy in 5 fractions)
100 % (50 Gy in 5 fractions)
50 % (45 Gy in 5 fractions)
2 years OS
85 % operable
61 % inoperable
16 % chest wall pain (grade 1–3)
3 % grade 2 pneumonitis
Modh et al. MSKCC (IJROBP 2013)
107 central tumors (83 primary NSCLC, 10 recurrent, 14 metastatic)
45–50 Gy in 4–5 Gx
2 years LC/OS 72 %/56 %
12 % grade ≥ 3
111 patients (100 primary NSCLC, 11 metastatic)
Volume-adapted iSABR (GTV < 12 ml → BED < 100, GTV ≥ 12 ml → BED ≥ 100)
18–30 Gy in 1 fraction, 50–60 Gy in 4–5 fractions
1 year LC/OS
94 %/90 % in primary NSCLC subgroup,
15 % DM rate,
no difference in LC/OS by BED
4.% grade 3
Taremi et al. Canada (IJROBP 2012)
108 patients, stage I NSCLC medically inoperable, 24 % w/o path diagnosis
Peripheral tumors 48 Gy in 4 fractions or 54–60 in 3 fractions, central tumors 50–60 Gy in 8–10 fractions
4 years LC/OS 92 %/30 %
4 years distant DFS 83 %
11 % grade 3
Lagerwaard et al. VUMC (IJROBP 2008)
206 patients T1-2N0
Risk-adapted 60 Gy in 3 fractions (T1), 5 fractions (T1 near CW or T2), or 8 fractions (central)
2 years LC/OS 83 %/64 %
(LC 92 % T1, 71 % T2)
2 years distant PFS 77 %
3 % grade ≥3 pneumonitis
2 % rib fracture
1 % late CW pain
Badiyan et al. Wash U. (RO 2013)
120 patients (early-stage NSCLC and AIS)
54 Gy in 3 fractions
3 years LC/OS
100 %/35 % AIS ,
86 %/47 % NSCLC
Not reported
Bradley et al. Wash U. (IJROBP 2010)
91 patients stage I/II NSCLC, medically inoperable
Peripheral tumors 54 Gy in 3 fractions, central tumors 45 Gy in 5 fractions
2 years LC/OS
86 %/70 %
3 % grade 2 pneumonitis
4 % rib fracture
1 % brachial plexopathy
Palma et al. VUMC (IJROBP 2012)
176 patients stage I NSCLC, severe COPD
60 Gy in 3–5 fractions
3 years LC/OS
89 %/47 %
3 % grade 3
Chang et al. MDACC (RO 2012)
130 patients stage I NSCLC
50 Gy in 4 fractions
2 years LC/OS
98 %/78 %
12 % grade 2–3 pneumonitis
Griffioen et al. VUMC (RO 2013)
62 patients with multiple synchronous primary early-stage NSCLC
54–60 Gy in 3–8 fractions
2 years LC/OS
84 %/56 %
4.8 % grade 3
Role as Boost for Locally Advanced Lung Cancer
Studies have suggested a role for dose escalation as part of conventional chemoradiation in locally advanced lung cancer , with current focus on reduced volume boost , to minimize normal lung toxicity, for which SBRT may be of utility.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree