7
Lung and Central Airway Malignancies
Sarah Baker and Alysa Fairchild
INTRODUCTION
Lung cancer remains the leading cause of cancer mortality worldwide, with 1.8 million patients diagnosed in 2012.1 75% to 85% of patients with lung cancer present with disease too advanced to be treated with curative intent.2 The majority of patients with advanced lung cancer have symptoms amenable to palliation with radiation therapy (RT)3; for example, 20% to 30% will develop airway obstruction at some point during their disease course.4 Additionally, lung metastases develop in 20% to 54% of extrathoracic malignancies, commonly from breast and colorectal primaries, and may also become symptomatic enough to require RT.5
Palliative thoracic RT is therefore a key component of the care of many patients. RT can be indicated in the clinical scenarios of airway obstruction, hemoptysis, cough, chest pain, dyspnea, hoarseness, Horner’s syndrome, and superior vena cava obstruction (SVCO), contributing to improvements in quality of life (QoL). This chapter summarizes the literature surrounding dose and fractionation, techniques, toxicities, and outcomes of palliative thoracic RT delivered for these common indications, as well as briefly describes essential supportive care considerations. Although the majority of the available literature describes non–small-cell lung cancer (NSCLC), many aspects of the following chapter, such as pretreatment optimization and technical factors, are applicable to the palliative treatment of small cell lung cancer (SCLC) and lung metastases. Consolidative thoracic RT after systemic therapy in extensive stage SCLC will also be discussed.
WHICH PATIENTS WITH STAGE IV NSCLC CAN BE TREATED AGGRESSIVELY?
While patients with metastatic disease are nearly always only candidates for palliative-intent treatment, there is a small proportion of patients with stage IV NSCLC—approximately 7%6—who either present with oligometastases or are rendered oligometastatic after palliative systemic therapy. While the specific definition of oligometastases varies, it is often taken as fewer than five discrete lesions outside of the primary site.7 High-dose local RT (typically delivered as stereotactic body radiation therapy [SBRT]) results in high treated metastasis control rates, delaying progression, and allowing continued systemic treatment. However, the topic of SBRT for oligometastases is beyond the scope of this chapter as the goal of RT in that situation is long-term disease control rather than palliation of symptoms. Excellent review articles on SBRT of lung metastases are available.8–10
WHICH PATIENTS WITH STAGE III NSCLC CANNOT BE TREATED AGGRESSIVELY?
The most robust prognostic factors in NSCLC are stage of disease, involuntary weight loss over the previous 3 to 6 months, and performance status (PS).10 In general, patients with Eastern Cooperative Oncology Group (ECOG) PS greater than 2, weight loss greater than 10% over the last 3 months, or tumor size greater than 8 cm11,12 are not likely to tolerate radical treatment—either surgery or definitive RT.13 In patients with stage III disease with poor prognostic factors who cannot be treated for cure, a recent phase III trial investigated combined modality therapy, which incorporated hypofractionated RT. A total of 191 patients were randomized to concurrent chemoradiotherapy (42 Gy in 15 fractions starting with cycle two of chemotherapy) versus carboplatin and vinorelbine alone. The combined modality arm had significantly improved median overall survival (12.6 vs. 9.7 months) and long term QoL, but experienced more frequent hospital admissions (49% vs. 25%; P < .01) for treatment-related side effects including esophagitis and infection.14
EMERGENCY RADIATION THERAPY FOR SVCO AND AIRWAY COMPROMISE
SVCO from direct invasion or extrinsic compression is attributable to lung cancer 50 to 70% of the time.15–18 Ten percent of all patients with SCLC, and 2% to 4% of those with NSCLC, will develop SVCO at some point in their disease trajectories.15–18 Thrombosis as a contributor to SVCO syndrome is becoming more common due to the increased prevalence of intravascular devices such as catheters and pacemakers,15,19 and it often coexists with malignancy-associated obstruction due to vascular hold-up. If thrombus is present, anticoagulation should be initiated,20 but there is no evidence supporting its prophylactic use in the absence of other indications. Additional considerations include the placement of an endovenous stent, usually by interventional radiology, followed by RT; it is unclear at present whether long-term anticoagulation is also required.21
For more proximal mainstem or tracheal obstruction, especially in the context of stridor, rapid debulking can be provided through bronchoscopic ablation or laser, where resources exist, followed by urgent RT.22–24 Occasionally, stenting, intubation, or tracheostomy may be required. Intraluminal stents provide symptom relief approximately 90% of the time.23,25–27
In cases of severe respiratory symptoms including stridor, patients require stabilization before RT to decrease the risk of acute decompensation on the treatment couch, and to mitigate symptoms in the interval before expected onset of benefit from RT, which ranges anywhere from 72 hours to a more typical 1 to 2 weeks.22 RT-induced edema can transiently exacerbate symptoms. While there is no level 1 evidence supporting steroid use for airway obstruction or SVCO, steroids may temporize the transient worsening of symptoms due to treatment, preventing a serious situation from becoming disastrous.
WHAT FIRST: PALLIATIVE RADIATION THERAPY OR PALLIATIVE SYSTEMIC THERAPY?
Both in general and in the setting of SVCO specifically, the addition of concurrent chemotherapy has not been conclusively shown to improve response rates, locoregional control, symptomatic relief, or survival.28–31 Therefore, palliative RT and palliative systemic therapy are almost always given sequentially, with advantages and disadvantages to each approach (Table 7.1).
Assuming no indication exists for emergency thoracic RT, patients ineligible for curative-intent treatment should be reviewed in a multidisciplinary rounds setting, so a consensus recommendation on an appropriate sequence of therapy can be reached (Table 7.2). This is essential, especially with a nonsquamous NSCLC patient with a tumor that is epidermal growth factor receptor- or anaplastic lymphoma kinase-mutated, which carry additional options for therapeutic intervention. The importance of obtaining a tissue biopsy prior to initiation of therapy cannot be overstated.
WHO SHOULD NOT BE TREATED WITH PALLIATIVE THORACIC RADIATION THERAPY?
Patients with an estimated life expectancy less than 1 month are not expected to derive substantial symptom improvement from thoracic RT, which often takes 4 to 6 weeks for full benefits to manifest. The time, inconvenience, and potential acute side effects of RT are not offset by significant improvements in symptomatology in that situation, and best supportive care should be the approach of choice. The exception is hemoptysis, which will often respond or resolve within a few days.37
TABLE 7.1 Advantages and Disadvantages of RT and Systemic Therapy as Initial Palliative Treatment Modalities

TABLE 7.2 Patient Selection for Palliative Thoracic RT: Factors to Consider
General |
|
Patient factors |
|
Disease factors |
|
PS, performance status; RT, radiation therapy; SVCO, superior vena cava obstruction.
Thoracic RT in asymptomatic but incurable NSCLC provides no survival benefit and does not definitively prevent future development of symptoms, and may in fact reduce short term QoL due to acute side effects.32,33 The risks and benefits of short course RT should be discussed with the patient, as proceeding is likely worthwhile only in the situation of impending airway, neurological, or vascular compromise (Table 7.3).
Patients with confusion (delirium or dementia) may not be able to tolerate coming to the cancer center for multiple daily visits. Short fractionation regimens and simple setup positions should be employed with additional immobilization measures as required. Assessment of ability to give informed consent is essential.
Although no specific criteria exist for minimum pre-RT pulmonary function, patients with poor lung function have less resilience for lung toxicity, and any complications that do occur may be more serious and potentially life-threatening. Baseline pulmonary function testing should be performed if the patient can tolerate them, and RT should be delivered with caution if the FEV1 and/or diffusing capacity of the lung for carbon monoxide (DLCO) are less than 20% of predicted values for age and gender. The need for supplemental oxygen is not a contraindication for palliative lung RT, especially when required for a reversible etiology such as a pulmonary embolus or pneumonia. However, degree of both dyspnea and orthopnea should be evaluated at the time of the initial physical examination to ensure these symptoms will not compromise a patient’s ability to lie supine and still. Arrangements for continuous oxygen during set-up and treatment will help increase patient comfort.
TABLE 7.3 Advantages and Disadvantages of Different Palliative RT Schedules

It should be noted that patients should not be deemed poor RT candidates on the basis of advanced age alone. Surveillance, Epidemiology, and End Results Medicare linked data suggests an age-related disparity in the receipt of palliative RT in the United States, even after controlling for confounding covariates such as PS.38 However, when controlling for stage and PS, age is not an independent predictor of worse outcome, nor are older patients less likely to complete a prescribed course of palliative thoracic RT.39
PRERADIATION THERAPY OPTIMIZATION
Several steps may be taken to optimize a patient’s status prior to initiation of therapy, to increase their comfort as well as the likelihood of completing the RT, such as short course steroids or supplemental oxygen (Table 7.2). Consider thoracentesis for pleural effusion, and treating acute concurrent illness such as postobstructive or aspiration pneumonia. Abdominal distension can reduce lung volumes and contribute to dyspnea and can be alleviated by treating constipation or performing paracentesis for ascites. Medical optimization of active comorbid illnesses such as congestive heart failure (CHF) or chronic obstructive pulmonary disease (COPD) via appropriate specialist referral may be helpful, especially if it can be performed without undue delay. For patients with pain, a short-acting opioid can ease transfer on and off the simulation and treatment couch, and help ensure that patients are comfortable enough to remain still during treatment. Opioids are also useful to address breathlessness and cough. Anxiolytics could be considered for anxiety or claustrophobia associated with an immobilization shell.
Case 7.1: Radiation Therapy in the Setting of Ventilation
A 75-year-old woman with biopsy-confirmed stage IV NSCLC presents to her radiation oncology consultation in respiratory distress. She is agitated, using accessory muscles of respiration with three to four word dyspnea despite 4 L of oxygen. She has not been able to take in solid food for a number of weeks due to significant dysphagia, requiring emergency department visits for intravenous hydration, most recently 48 hours prior. She has lost 40 lb. in the last 3 months. On enhanced CT scan of the chest, there is an 8 cm mediastinal mass invading the esophagus and compressing both mainstem bronchi. She is stage IV on the basis of contralateral pulmonary nodules consistent with metastatic lesions and ECOG PS is 3 to 4.
What are the appropriate initial steps with this patient?
![]() Ensure that the patient is stable by performing vital signs in the clinic.
Ensure that the patient is stable by performing vital signs in the clinic.
![]() Admit for symptom control including oxygen titration, opioids, nutritional bypass (feeding tube placement, if appropriate), and initiation of steroids. Consider a proton pump inhibitor or H2 blocker for gastric protection as well as inhaled bronchodilators. Proceeding with RT immediately could exacerbate her respiratory distress due to radiation-induced swelling.
Admit for symptom control including oxygen titration, opioids, nutritional bypass (feeding tube placement, if appropriate), and initiation of steroids. Consider a proton pump inhibitor or H2 blocker for gastric protection as well as inhaled bronchodilators. Proceeding with RT immediately could exacerbate her respiratory distress due to radiation-induced swelling.
![]() Palliative care consultation should be undertaken.
Palliative care consultation should be undertaken.
Despite the aforementioned interventions, over the next 2 days, the patient’s breathing worsens and her oxygen saturation falls to 81% to 84% on 15 L.
What is the optimal management?
![]() Transfer to an acute care setting for stenting of the left mainstem bronchus. After the procedure, she remained intubated and ventilated in the Intensive Care Unit.
Transfer to an acute care setting for stenting of the left mainstem bronchus. After the procedure, she remained intubated and ventilated in the Intensive Care Unit.

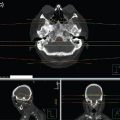
FIGURE 7.1 Case 1 contours and dose distribution. After insertion of a left mainstem bronchus stent, the patient received the first four fractions of emergency thoracic RT while intubated. Red: GTV. Cyan: PTV. Beam arrangement: parallel opposed. Energy: 6MV. Beam modifiers: multilieaf collimation. Dose fractionation schedule: 30 Gy/10 on consecutive days including the weekend.
If the patient is stable, can she proceed to RT?
![]() Palliative RT can be initiated while intubated, and this patient completed 4 of 10 planned fractions in this manner, transported on a daily basis between hospitals (Figure 7.1). After extubation, she completed the prescribed 30 Gy in 10 fraction course without difficulty.
Palliative RT can be initiated while intubated, and this patient completed 4 of 10 planned fractions in this manner, transported on a daily basis between hospitals (Figure 7.1). After extubation, she completed the prescribed 30 Gy in 10 fraction course without difficulty.
![]() By the 2 month follow-up visit, she was no longer on supplemental oxygen, denied dyspnea or dysphagia, and was regaining weight. She went on to receive four cycles of cytotoxic systemic therapy with an excellent clinical and radiographic response.
By the 2 month follow-up visit, she was no longer on supplemental oxygen, denied dyspnea or dysphagia, and was regaining weight. She went on to receive four cycles of cytotoxic systemic therapy with an excellent clinical and radiographic response.
OUTCOMES AFTER PALLIATIVE THORACIC RADIATION THERAPY
The dose required for optimal symptom palliation and whether higher doses improve survival or provide more durable local control are issues that have been addressed in several randomized controlled trials (RCTs). Table 7.4 summarizes the results of a recent meta-analyses.
In general, thoracic RT provides effective symptom palliation for hemoptysis, cough, and chest pain (Table 7.5). Dyspnea can be more refractory to RT as it is often multifactorial; in fact, up to 25% of dyspenic cancer patients lack obvious lung involvement.45 Atelectasis secondary to airway obstruction and symptoms related to neurological compromise such as hoarseness may not normalize following RT, particularly if present for an extended duration prior to treatment.
TABLE 7.4 Meta-analyses of Dose Fractionation Schedules for Palliative Thoracic RT

TABLE 7.5 Symptom Response After Palliative Thoracic RT. Refers to NSCLC Unless Stated Otherwise

The onset of symptom improvement varies from several days in the case of hemoptysis to approximately 2 to 4 weeks for most other symptoms.22,46,47 RT-induced edema can exacerbate cough and dyspnea for the first 1 to 2 weeks following treatment, particularly with more hypofractionated schedules such as 17 Gy/2 fractions one week apart or 10 Gy/1 fraction.46,47 While one or two fraction regimens may be associated with acute esophagitis, they may also contribute to more rapid onset of palliation.46,47 A retrospective review of SVCO due to any histology reported that 3 Gy or more per fraction resulted in higher rates of symptom relief at 2 weeks than conventional fractionation.29
The duration of symptom improvement is a difficult endpoint to ascertain with certainty due to varying follow-up schedules, heterogeneous (or absent) symptom assessment, and poor patient survival overall. Reports describe improvements in total symptom score lasting approximately 22 weeks following RT,46 to lasting at least 50% of the patient’s remaining survival.43,44,48 There is no strong evidence that higher dose schedules result in longer lasting control of symptoms.40
Case 7.2: Radiation Therapy for Superior Vena Cava Obstruction
A 71-year-old woman with a sustained complete response to treatment 5 years prior for a stage IV follicular non-Hodgkin lymphoma presents with bilateral arm and neck swelling, headache, cough, and dyspnea. She is not in respiratory distress. Enhanced CT of the chest and upper abdomen demonstrates an 11 cm heterogeneous right upper lobe mass causing almost complete obstruction of the SVC. There is dilation of the azygous venous system with extensive superficial collateral veins apparent. Transthoracic needle core biopsy confirms poorly differentiated adenocarcinoma.
What treatment should she receive?
![]() She is stable and does not require emergency stent placement or airway stabilization.
She is stable and does not require emergency stent placement or airway stabilization.
![]() She was treated with 20 Gy/5 fractions (Figure 7.2) and experienced complete resolution of her symptoms 6 days after completing RT.
She was treated with 20 Gy/5 fractions (Figure 7.2) and experienced complete resolution of her symptoms 6 days after completing RT.

FIGURE 7.2 Example of treatment planning for SVCO. Beam arrangement: parallel opposed. Energy: 15 MV. Beam modifiers: multilieaf collimation. Dose fractionation schedule: 20 Gy/5 fractions on consecutive days including the weekend. Weekend treatment may not be necessary at these fraction sizes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree