■
Lung Transplantation
Since the first lung transplantation was performed in 1963, there have been major improvements in the surgical technique and posttransplantation management of organ recipients. Lung transplantation represents the only effective therapeutic option for many patients with advanced lung disease. Although there continue to be advancements in the understanding of the posttransplantation care of these patients, improvements in long-term survival have been limited by complications, primarily chronic allograft dysfunction. Radiology plays a major role in the detection and characterization of transplantation complications. The goal here is to discuss the imaging findings of these complications and the role of radiology in the multidisciplinary care of lung transplant recipients.
Determining Who Gets a Lung Transplant
In 2013, approximately 1900 lung transplantations were performed in the United States according to the Organ Procurement and Transplantation network. This represents a relatively small number compared to other organs including kidney (~17,000) and liver (~6500). Lung transplantation is performed in patients with advanced lung disease from a variety of causes ( Table 41.1 ). Trends over the past decade have shown increased utilization of lung transplantation in interstitial lung disease patients, primarily idiopathic pulmonary fibrosis, compared to those with chronic obstructive pulmonary disease.
| INDICATION | LUNG TRANSPLANTS (%) |
|---|---|
| Chronic obstructive pulmonary disease (excluding alpha-1 antitrypsin deficiency) | 33.5 |
| Interstitial lung disease | 23.7 |
| Cystic fibrosis | 16.6 |
| Alpha-1 antitrypsin deficiency | 5.8 |
| Idiopathic pulmonary arterial hypertension | 3.1 |
| Sarcoidosis | 2.5 |
| Connective tissue disease | 1.3 |
| Lymphangioleiomyomatosis | 1.0 |
Selection Process
Lung transplant recipients are chosen based on their lung allocation score. This system was developed in 2005 in an attempt to prioritize waiting list candidates based on their need and expected posttransplantation survival. Priority is given to patients who are likely to have a significantly greater 1-year survival with a transplant compared to a patient without a transplant. Some of the variables included in the lung allocation score include age, body mass index, diagnosis, lung function (i.e., forced vital capacity), need for oxygen, P co 2 levels, pulmonary artery systolic pressure, and the presence of comorbidities such as diabetes and renal failure.
Some absolute contraindications to lung transplantation include a history of malignancy (nonskin) in the past 2 years, untreatable nonlung major organ dysfunction, noncurable infection, such as viral hepatitis B, and significant chest wall deformity.
Problem Solving With CT and PET
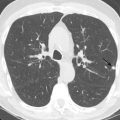
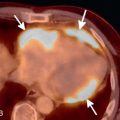
Radiology is valuable in screening for lung cancer prior to transplantation. Patients with advanced lung disease have a significantly higher incidence of bronchogenic carcinoma compared to the population as a whole. Volumetric CT of the chest is routinely performed in candidates when their lung allocation score predicts a reasonable likelihood of transplantation in the near future. It is important to note that bronchogenic carcinoma may have an atypical radiographic appearance in patients with advanced lung disease because of the significant architectural distortion of the lung ( Fig. 41.1 ). Any persistent focal lung opacity should be viewed as possible malignancy. 18 F-fluorodeoxyglucose (FDG) PET is useful in patients with indeterminate CT abnormalities and is particularly helpful in distinguishing malignancy from scar in patients with fibrotic lung disease.
How a Lung Transplantation Is Performed
A bilateral or single lung transplantation may be performed. A bilateral transplantation is required for patients with chronic infection, such as those with cystic fibrosis. Bilateral lung transplantation is also preferred at many institutions for all patients because there is evidence to suggest improved survival compared to a single lung transplant. Single lung transplantation is preferred for patients who are at a higher surgical risk, such as older patients. It also may be performed when complications, such as bleeding, necessitate early termination of the surgery.
The surgical procedure is performed one lung at a time. The pulmonary veins, pulmonary artery, and bronchus are divided. These are then reanastomosed to the allograft, typically in reverse order. Of note, the lymphatic, bronchial arterial, and neurogenic connections are not reestablished in the allograft. Cardiopulmonary bypass may be required in selected patients.
Common Surgical Complications
Major postoperative complications have become less common, given advances in technique over the past several decades. The most common complications include postoperative pleural hematomas, sternal dehiscence, and anastomotic issues.
Problem Solving With Chest X-Ray and CT
Postoperative pleural hematomas may be seen in the first few days after surgery and are more common in patients with significant pretransplantation pleural inflammation and adhesion formation, as in patients with cystic fibrosis. Pleural hematomas are usually discovered on chest radiographs and manifest as a significant pleural collection that develops immediately or within the first few days after surgery.
Sternal dehiscence is often the result of infection resulting in weakening of the bone. It usually occurs within the first few months after transplantation. Typical CT findings include separation of sternal fragments, areas of bone lysis, and significant fluid collections adjacent to the sternum. Sagittal and coronal reformats may be helpful in visualizing the distortion of the three-dimensional structure of the sternum.
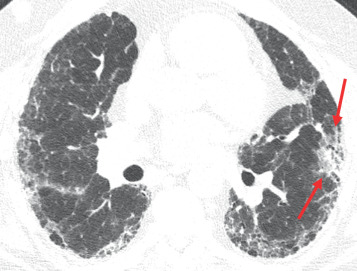
Anastomotic breakdown usually involves the bronchial connection and is typically seen in the first month after transplantation. Pneumomediastinum and/or pneumothorax may indicate that the anastomosis is not intact. Postoperative air in the mediastinum is a normal finding in the first several weeks after surgery, but a predominance of air around the bronchial anastomosis should raise concern for an anastomotic dehiscence ( Fig. 41.2 ). Additionally, significant mediastinal air after the first few weeks is also suggestive. Extraluminal air should not be confused with the normal finding of a telescoped bronchial anastomosis.

Breakdown of the pulmonary arterial or venous anastomoses is rare, but has a high rate of mortality. This manifests as saccular extraluminal collections of contrast at anastomotic sites. Stenoses may also occur at the vascular anastomoses.
Typical Medical Therapy After Transplantation and Normal Surveillance Regimens
After receiving a lung transplant, recipients are treated with lifelong immunosuppression. The lung is unique because of its constant exposure to infectious agents in the environment; thus immunosuppression is a balance between the risk of infection and rejection. Typical regimens include a combination of a calcineurin inhibitor (e.g., tacrolimus), antimetabolite (e.g., mycophenolate mofetil), and corticosteroids. Asymptomatic patients undergo regular bronchoscopy, pulmonary function tests (PFTs), and CT imaging. This surveillance is most intensive in the first year posttransplantation and then subsequently falls off in regularity. Patients with any symptom will also undergo a similar diagnostic evaluation.
Problem Solving With Chest X-Ray and CT
Routine imaging surveillance may be performed with volumetric CT or axial high-resolution CT (HRCT). Axial HRCT with skipped sections is sensitive for most abnormalities and offers the advantage of imparting a lower radiation exposure. It is appropriate in the surveillance of common complications such as infection, acute rejection, and chronic rejection. Volumetric CT is more sensitive for focal complications, such as bronchial strictures and malignancies of the native lung. At institutions where axial HRCT is the preferred routine imaging test, volumetric CT should be performed periodically to assess for focal abnormalities.
Most Common Complications of Lung Transplantation and the Role of Imaging in Monitoring These Complications
Complications in lung transplantation may be due to the surgery itself, the immunosuppression regimen, or an immune reaction against the allograft. Diagnosis of these complications is a multidisciplinary endeavor that incorporates clinical symptoms, laboratory findings, bronchoscopic findings, pathologic information, and radiology. Radiology is a key component in the detection and characterization of abnormalities. Complications tend to occur at predictable intervals after transplantation, and imaging findings must always be interpreted in this context ( Table 41.2 ). The role of imaging includes the following.
| TIMING | COMPLICATION | COMMENTS |
|---|---|---|
| Postoperative period | Primary graft dysfunction | Most common cause of diffuse opacities within first 72 h |
| Hyperacute rejection | Immediate reaction from preformed antibodies; rare | |
| Venous anastomotic stenosis | Rare | |
| Pleural hematoma | Significant pleural collection appearing within first few days | |
| Early complications (typically within first year) | Infection | Bacterial and fungal infections peak at 1 month; viral infections peak at 150 days. |
| Acute cellar rejection | Initial episode usually in first year, but recurrent episodes may occur for years after | |
| Anastomotic issues | Stenosis or dehiscence of bronchus, artery or vein | |
| Bronchial strictures | Usually occurs 2–10 months after transplantation | |
| Posttransplantation lymphoproliferative disease | Peak incidence at 1 year | |
| Late complications (typically after first year) | Infection | Common cause of abnormalities throughout the life of the transplant |
| Chronic rejection | Most common cause of mortality, usually after 1 year | |
| Recurrence of primary disease | May be early or late | |
| Complications in native lung (e.g., malignancy or infection) | May be early or late |
Role of Imaging
Detection
Imaging may detect complications prior to their becoming clinically occult. In some cases, radiology may be more sensitive than clinical symptoms or abnormalities on PFTs. By detecting complications early, treatment can be initiated to prevent further injury.
Characterization
Complications may display overlapping clinical, laboratory, and PFT abnormalities. Radiology adds significant additional information in the determination of which complication is present. For example, both chronic allograft dysfunction and a bronchial stricture may cause dyspnea and airflow obstruction on PFTs; however, these are easily distinguished on imaging.
Assessing Response to Treatment
Radiology is accurate in determining the extent and severity of complications. It provides a global assessment of lung abnormality and thus can determine if there has been an appropriate response to treatment on interval follow-up imaging.
Primary Graft Dysfunction
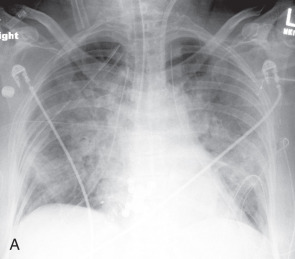
Primary graft dysfunction (PGD) is a form of acute lung injury that occurs within 72 hours of transplantation. It is a result of multiple factors relating to the harvesting and reimplantation of the graft, including ischemic injury, the preservation process, and reperfusion on reimplantation. PGD replaces previously used terms such as reperfusion edema, reimplantation edema, and primary graft failure. PGD is seen in 10% to 50% of lung transplant recipients and is the most common cause of early mortality. It also predisposes patients to the development of late complications, namely chronic allograft dysfunction. It is graded (0–3) using two parameters, the oxygenation (ratio of Pa o 2 /Fi o 2 ) and the presence or absence of radiographic infiltrates consistent with pulmonary edema.
Problem Solving With Chest X-Ray and CT
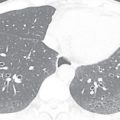
Typical radiographic or CT features of PGD include diffuse or symmetric bilateral consolidation and/or ground-glass opacities appearing within 72 hours after transplantation ( Fig. 41.3 ). Interlobular septal thickening on CT, representing interstitial edema, is also commonly seen. To make a diagnosis of PGD, other causes of diffuse pulmonary opacities should be excluded, including hyperacute rejection, venous anastomotic obstruction, cardiogenic pulmonary edema, and pneumonia. Abnormalities are most severe within the first 2 weeks after lung transplantation, but typically show significant improvement by at least the 12th week.

Infection
The constant exposure of the allograft to the environment puts lung transplant patients at a particularly high risk of infection. Also, the immunosuppressive regimen they receive is particularly intensive when compared to most other solid organ transplantations. The titration of this regimen is a constant balance between the risks of infection and rejection.
Other features that may predispose patients to infection include denervation of the graft, causing a diminished cough reflex, impaired lymphatic drainage, and lack of a bronchial arterial supply. Infection is the leading cause of mortality between 31 days and 1 year posttransplantation, but remains a common cause of morbidity and mortality throughout the life of the recipient.
Problem Solving With Chest X-Ray and CT
The radiographic findings of infection mirror those seen in nontransplanted patients. There is significant overlap in the radiographic findings of different organisms, so imaging needs to be interpreted in the context of clinical and laboratory findings. A comprehensive review of the imaging findings of infection is covered in Chapter 16 ; however, it is important that the radiologist understand issues specific to lung transplant patients and which specific CT patterns are particularly suggestive of certain categories of organisms.
Bacterial infection is the most common infection overall. Its incidence peaks in the early period, approximately 1 month posttransplantation. Unilateral or bilateral asymmetric consolidation, with or without centrilobular nodules and tree-in-bud opacities, is usually due to bacterial infection, particularly when seen in this early period.
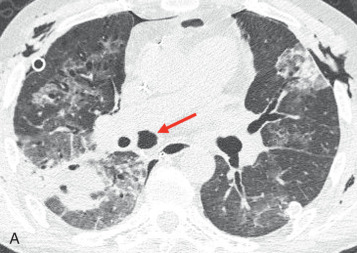
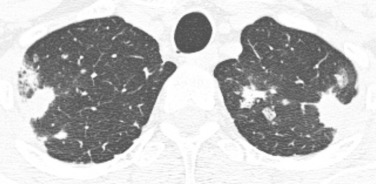
Bilateral large nodules or rounded regions of consolidation with irregular margins are most suggestive of fungal infection, particularly Aspergillus ( Fig. 41.4 ). Fungal infections are the second most common type of infection and tend to peak early after transplantation, similar to bacterial infections.
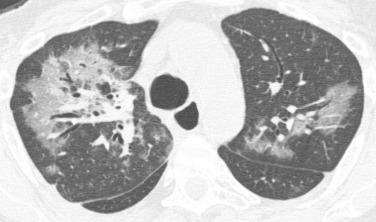
Diffuse or bilateral symmetric ground-glass opacities are usually due to viral infection ( Fig. 41.5 ). Consolidation, nodules, and tree-in-bud opacities may also be seen, but the symmetric distribution and presence of significant ground-glass opacity should specifically suggest a viral cause. The peak incidence of viral infection occurs later than bacterial and fungal infections, around 150 days posttransplantation. Viral infections are particularly important given their predisposition to induce chronic allograft dysfunction (bronchiolitis obliterans). Because of this association, many centers will treat an acute viral infection with an increase in the antirejection regimen. It is important to note that viral infection cannot be distinguished from acute cellular rejection (ACS) based on imaging alone. Cytomegalovirus pneumonia is the most common pathogen, although universal prophylaxis against cytomegalovirus has led to a decreased incidence.
Other infections, including mycobacterial, Pneumocystis jiroveci, and Nocardia, may be seen in lung transplantation patients, but are much less common than the others discussed above. Nontuberculous mycobacterial infections are more common in cystic fibrosis patients. Mycobacterium abscessus is a particularly virulent pathogen and is a relative contraindication to transplantation when discovered. Its CT features overlap with those of other nontuberculous mycobacterial infections; these include centrilobular nodules, tree-in-bud opacities, bronchiectasis, and cavities.
Rejection
There are different types of rejection after lung transplantation.
Antibody-Mediated and Hyperacute Rejection
Antibody-mediated rejection (AMR) is rare and occurs when the humoral immune system targets the allograft. This is in contrast to ACS, a predominantly T cell– mediated reaction. When AMR occurs immediately after transplantation, it is due to preexisting antibodies in the recipient and is called a hyperacute rejection. The predominant pathologic manifestation is acute lung injury, and loss of the allograft is common. The radiographic manifestations are indistinguishable from PGD and consist of diffuse or symmetric ground-glass opacities and consolidation that develop immediately after transplantation.
AMR may also occur later, often within the first year after transplantation, and is due to the interval development of an antibody response against the graft. Pathology shows diffuse alveolar damage, often with capillaritis. Radiographic manifestations are similar to those of hyperacute rejection, with diffuse or symmetric ground-glass opacities and consolidation. Patients who survive an episode of AMR are at high risk for developing bronchiolitis obliterans syndrome (BOS).
Acute Cellular Rejection
ACS affects more than 50% of lung transplant recipients. Although the first episode of ACS typically occurs within the first year after transplantation, patients may have recurrent episodes long after the initial 1-year period. ACS accounts for less than 5% of the mortality of lung transplantation but is the primary risk factor for the development of BOS. Histopathologically, ACS is characterized by perivascular mononuclear predominant inflammation, often accompanied by a lymphocytic bronchiolitis. The clinical manifestations are varied. It may be detected incidentally on surveillance bronchoscopy in asymptomatic patients or may be seen in patients with profound dyspnea and hypoxemia.
Bronchoscopy is the cornerstone of diagnosis in ACS. Findings on transbronchial biopsy are graded based on the severity of findings (0–4) and the form(s) of rejection present, including ACS (A), lymphocytic bronchiolitis (B), and obliterative bronchiolitis (C). Pulse dose corticosteroids are typically given to patients with mild cellular rejection (A2) or higher.
Problem Solving With CT
The most common CT findings of ACS are ground-glass opacities, consolidation, and septal thickening ( Fig. 41.6 ). The findings are typically bilateral and symmetric, but may occasionally be unilateral or asymmetric. Pleural effusion may be seen as the only manifestation of ACS or may be seen in association with other findings. These findings overlap significantly with infections, particularly viral infections, so bronchoscopy is the primary diagnostic test used to distinguish them.