Factor
EORTC (Ferme et al. 2007)
NCI-C (Meyer et al. 2012)
Number of nodal sites
≥3
≥4
≥4
LMA
Present
Present
Presenta
ESR & B-symptoms
≥50, no “B” symptoms
≥30, “B” symptoms
≥50, no “B” symptoms
≥30, “B” symptoms
ESR ≥ 50
Extranodal involvement
Present
Present
Age (years)
>50
≥40
Histology
MC/LD
MC/LD
Sex
Male
For patients with advanced HL, the International Prognostic Factors Project on Advanced Hodgkin’s Disease evaluated 5141 patients treated with combination chemotherapy, with or without radiation therapy, and identified seven factors that had independent prognostic significance for the outcome freedom from progression These included serum albumin, hemoglobin, male sex, stage, age, and white-cell count (Table 2). The relative risk of each of these factors was relatively similar and ranged from 1.26 (stage IV disease) to 1.49 (hypoalbuminemia). When patients were grouped into 6 prognostic categories based on number of adverse factors (0, 1, 2, 3, 4, and ≥5), 5-year freedom from progression was 84, 77, 67, 60, 51, and 42 % with a similar declining trend in overall survival (Fig. 1). Thus, while clinical factors were able to partition patients into risk groups, the relative differences between risk groups were modest.
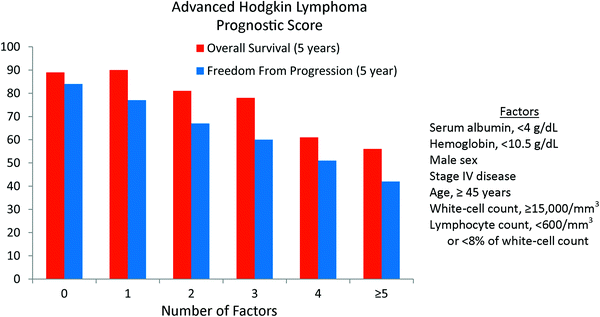
Table 2
International Prognostic Factor Project on Advanced Hodgkin’s Disease
Adverse prognostic factor | Relative risk |
|---|---|
Serum albumin, <4 g/dL | 1.49 |
White-cell count, ≥15,000/mm3 | 1.41 |
Age, ≥45 years | 1.39 |
Lymphocyte count, <600/mm3 or <8 % of white-cell count | 1.38 |
Male sex | 1.35 |
Hemoglobin, <10.5 g/dL | 1.35 |
Stage IV disease | 1.26 |

Fig. 1
Prognostic scoring system for advanced Hodgkin lymphoma by the International Prognostic Factors Project on Advanced Hodgkin’s Disease. The primary endpoint was 5-year freedom from progression (NEJM 1998; 339:1506)
Similar to early-stage HL, outcomes in advanced HL have improved with more effective combination chemotherapy regimens. Recent randomized trials have demonstrated excellent outcomes that are approaching those of patients with early-stage disease. For example, 5-year freedom from treatment failure was 88 % with 8 cycles of dose-escalated BEACOPP in the GHSG HD9 study (Engert et al. 2009), 85 % with 4 cycles of dose-escalated BEACOPP and 4 cycles of baseline dose BEACOPP in GHSG HD12 (Borchmann et al. 2011), and 89 % with 6 cycles of dose-escalated BEACOPP in GHSG HD15 (Engert et al. 2012). Radiation therapy was used in all three studies using different criteria.
2.2 Pathological Factors
NLPHL is a rare subtype of HL, accounting for ~5 % of cases. The rarity of this disease has precluded prospective studies, particularly randomized comparisons of differing treatment approaches. The optimal management, particularly for early-stage disease, is controversial and studies in the literature are conflicting (Chen et al. 2010; Nogova et al. 2005; Savage et al. 2011). For localized disease, most patients are treated currently with involved-field radiation therapy alone.
The larger studies that have been performed demonstrate that the risk of relapse after treatment for NLPHL is very similar to classical HL, though patients with classical HL tend to relapse earlier (Nogova et al. 2008; Diehl et al. 1999). However, patients with relapsed NLPHL tend to survive relapse better with a corresponding advantage in long-term overall survival. Indeed, toxicities of treatment are the primary cause of death in patients with NLPHL leading many oncologists to avoid treatment regimens with significant risk. As NLPHL is a CD20 positive lymphoma, ritixumab is often administered. Further studies are necessary to better understand the biology of this rare disease and identify the optimal treatment approach.
2.3 Positron Emission Tomography
Imaging modalities, positron emission tomography (PET) in particular, play a central role in the management of patients with lymphoma. In addition to assessing the extent of disease at diagnosis and for disease surveillance after definitive therapy, PET response also has prognostic impact for both HL and NHLs.
Both interim and post-treatment imaging response with PET have been shown to be a powerful prognostic factors in HL, in all stages of disease and multiple clinical scenarios. Two studies highlight the importance of disease status after chemotherapy but before consolidation radiation therapy. In a Stanford study of 81 patients (73 % with early-stage disease) receiving Stanford V chemotherapy (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, prednisone) and consolidation radiation therapy, the presence of PET positive disease after chemotherapy strongly predicted for relapse. Of the six patients who were still PET positive following chemotherapy, four relapses occurred, all within the radiation field (median dose- 33 Gy). Freedom from progression was 96 % in PET negative patients and 33 % in PET positive patients (p < 0.0003).
Investigators from the Dana Farber Cancer Institute observed similar findings after ABVD. Among 73 patients with HL (88 % with early-stage disease), 13 were PET positive after chemotherapy. There were four relapses among these 13 patients, three of which were in the radiation field (median dose- 36 Gy). Two-year failure-free survival was 69 and 95 % for PET positive and PET negative cohorts, respectively (p < 0.01). While a positive post-chemotherapy PET scan is associated with inferior outcomes, many patients remain disease-free after consolidation radiation therapy. As local failure at the PET positive site is the dominant pattern of failure, higher doses of radiation therapy may be required to sterilize persistent local disease.
Most patients with advanced HL receive chemotherapy alone and post-chemotherapy PET imaging is highly predictive of outcome. In a prospective study by Weihrauch et al. (2001), patients with HL received chemotherapy alone and were then observed. Of ten patients who were PET positive after chemotherapy, six relapses occurred, all confined to the originally involved site. Compared with the cohort of patients who were PET negative after chemotherapy, the disease-free survival at 1 year was significantly less (40 versus 95 %, p = 0.004).
Patients with advanced HL who remain PET positive after chemotherapy appear to be at significantly less risk of relapse when consolidation radiation therapy is administered. In GHSG HD15, patients underwent post-treatment computed tomography (CT) imaging after BEACOPP chemotherapy (Engert et al. 2012). If a residual mass measuring ≥2.5 cm was present then PET imaging was performed. Patients who achieved a complete response by CT imaging and those with residual masses that were PET negative did not receive consolidation radiation therapy. Patients with residual masses that were PET positive did receive RT (30 Gy). Progression-free survival at 4 years was 93 % for PET negative (who did not receive RT) and 86 % for PET positive (who did receive radiation therapy), respectively (p = 0.02). This compares favorably with the study by Weihrauch et al. (2001) where no additional treatment was pursued. Patterns of failure were not reported in GHSG HD15. As mentioned previously, higher doses of radiation therapy may be required in the setting of PET positive disease to achieve durable local control.
A positive interim PET also has prognostic significance, though whether the chemotherapy course should be altered if an early complete response is not achieved is unknown. If an interim PET could consistently and accurately identify those patients who would ultimately have refractory or relapsed disease, then this would be a helpful test. However, many patients with a positive interim PET who continue on their current regimen ultimately achieve a complete response and remain disease-free.
The most dramatic study showing the potential prognostic significance of interim PET is from Denmark. Among 77 patients mostly treated with ABVD for all stages of HL, 2-year progression-free survival was 96 % for those who were PET negative after 2 cycles of chemotherapy compared with 0 % for those who were PET positive (Hutchings et al. 2006). A study from Italy showed similar findings, with a 2-year progression-free survival of 95 and 13 % for PET negative and PET positive patients, respectively after ABVD +/− consolidation radiation therapy (Gallamini et al. 2007).
Most studies have not been this dramatic. For example, an Italian study of 304 patients demonstrated 9-year progression-free survival of 95 and 31 % in the presence of a positive and negative interim PET scan, respectively (Zinzani et al. 2012). A study from the Massachusetts General Hospital demonstrated no difference in 4-year progression-free survival for interim PET positive versus PET negative patients (87 versus 91 %, p = 0.57), but post-treatment PET was significant (54 versus 94 %, p < 0.001). This study only included early-stage patients without bulky disease who largely received combined modality therapy (Barnes et al. 2011). Thus, while interim PET imaging likely has prognostic implications, whether one should alter therapy based on the interim PET response is unclear.
A major issue is PET interpretation. Currently, PET scans are interpreted as positive or negative based on visual analysis alone (Juweid et al. 2007). This leads to some inter-observer and intra-observer variability. An objective method to interpret PET imaging has yet to be validated.
2.4 Treatment-Specific Factors
Chemotherapy is the standard initial treatment for patients with HL in all stages. In early-stage disease, multiple randomized trials have shown that consolidation radiation therapy decreases the risk of relapse (Aviles and Delgado 1998; Laskar et al. 2004; Meyer et al. 2012; Nachman et al. 2002; Noordijk et al. 2005; Pavlovsky et al. 1988; Picardi et al. 2007; Straus et al. 2004) and may improve survival (Franklin et al. 2005). In general, the absolute improvement in progression-free survival is ~10 %. Admittedly, most patients are cured with chemotherapy alone. Identifying the subgroup of patients with residual microscopic disease after chemotherapy that would most benefit from consolidation RT has been challenging.
Currently, most studies attempting to define the subset of patients who are most likely to benefit from consolidation radiation therapy utilize either post-treatment or interim PET imaging. In a study by Picardi et al. (2007), 160 patients with HL (all with disease ≥5 cm) received 6 cycles of VEBEP (vinblastine, etoposide, bleomycin, epirubicin, predisone). Those with a good (≥75 % size reduction) but incomplete response by CT imaging but who were negative by PET were randomized to consolidation radiation therapy (32 Gy) or observation. Despite being PET negative, consolidation radiation therapy decreased the risk of relapse. Event-free survival at 5 years was 96 versus 86 %, respectively (p = 0.03). Patients who achieved both a negative PET and CT (n = 70) were not randomized and only received chemotherapy. Of 60 patients with follow-up data, there were seven relapses. Thus, a negative post-treatment PET, even with a negative CT, may lack sufficient sensitivity to indicate a true disease-free state. Interim PET, done after 2–3 cycles, is also being examined, but interim analyses from an EORTC/GELA study (H10) have not been encouraging (Engert 2012). Numerous prospective phase II and phase III studies are currently ongoing by cooperative groups in both the United States and abroad examining the ability of PET to identify patients who would most benefit from consolidation radiation therapy.
Based on the available data, the most effective treatment for early-stage classical HL is combined modality therapy, with the chemotherapy regimen, number of cycles, and dose of radiation therapy dependent on the initial presentation. The standard for advanced HL is chemotherapy. The role of radiation therapy is controversial with conflicting results from randomized studies (Fabian et al. 1994; Aleman et al. 2003).
3 Diffuse Large B-cell Lymphoma
3.1 Clinical Factors
Using data from 2,031 patients with aggressive NHL, two prognostic models were developed as part of the International Non-Hodgkin’s Lymphoma Prognostic Factors Project (The International Non-Hodgkin’s Lymphoma Prognostic Factors Project 1993). The first model was termed the International Prognostic Index (IPI) and included all patients. Five factors were found to be independently associated with survival including age, performance status, stage, number of extranodal sites, and LDH (Table 3). The relative risks of these factors were relatively low, ranging from 1.47 for stage to 1.96 for age. Five-year survival was 73, 51, 43, and 26 % when 0–1, 2, 3, and 4–5 risk factors were present (Fig. 2).
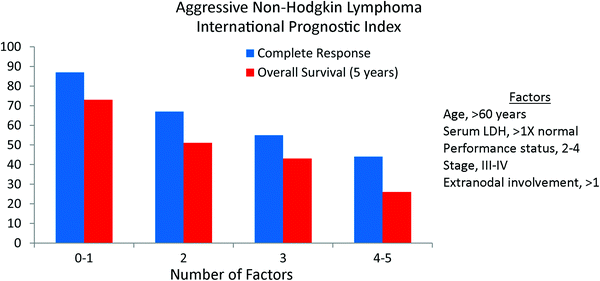
Table 3
International Non-Hodgkin’s Lymphoma Prognostic Factors Project
Adverse prognostic factor | Relative risk |
|---|---|
All patients (n = 1385) | |
Age, >60 years | 1.96 |
Serum LDH, >1X normal | 1.85 |
Performance status, ECOG 2-4 | 1.80 |
Extranodal involvement, >1 site | 1.48 |
Stage, III-IV | 1.47 |
Patients ≤60 years old (n = 885) | |
Stage, III-IV | 2.17 |
Serum LDH, >1X normal | 1.95 |
Performance status, ECOG 2-4 | 1.81 |

Fig. 2
The International Prognostic Index (IPI) for aggressive non-Hodgkin lymphoma by the International Non-Hodgkin’s Lymphoma Prognostic Factors Project. The primary endpoint was 5-year overall survival (NEJM 1993; 329:987)
As age was the most significant risk factor, and 60 is a common age limit for patients receiving more intensive regimens, an Age Adjusted IPI was also developed. When patients were divided by age, three factors remained independently significant- performance status, stage, and lactate dehydrogenase (LDH) (Fig. 3).
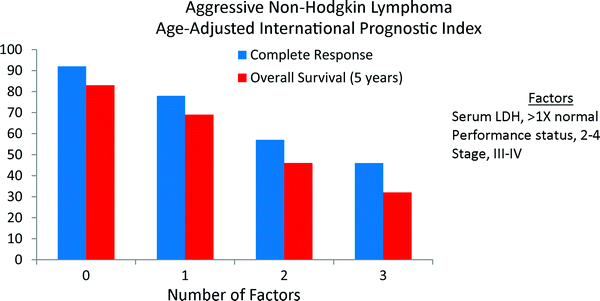

Fig. 3
The age-adjusted (≤60 years old) International Prognostic Index (IPI) for aggressive non-Hodgkin lymphoma by the International Non-Hodgkin’s Lymphoma Prognostic Factors Project. The primary endpoint was 5-year overall survival (NEJM 1993; 329:987)
The IPI was designed in the pre-rituximab era. In the last 10–15 years, multiple randomized studies have shown that rituximab significantly improves survival when combined with standard combination chemotherapy (Feugier et al. 2005; Pfreundschuh et al. 2006; Habermann et al. 2006). Using data from three prospective trials, the German High-Grade Non-Hodgkin’s Lymphoma Study Group found the IPI to retain prognostic significance in the rituximab era (Ziepert et al. 2010).
3.2 Pathological Factors
Classification of lymphomas continues to be based largely on histology and immunohistochemistry. With DLBCL, the heterogeneity of disease presentations and differences in response to therapy indicate that underlying biology plays a dominant role. Similar to breast cancer, the principal genetic changes responsible for this biological diversity are slowly being unraveled and have confirmed that DLBCL is a heterogeneous disease.
It was first reported by Alizadeh et al. (2000) that distinct subtypes of DLBCL could be identified using gene expression profiling, and that these subtypes were derived from different cells of origin. Germinal center B-cell (GCB) subtype appears to derive from cells actively involved in somatic hypermutation. Lymphomas within this category have a higher prevalence of mutations in pathways involved in apoptosis, often harbor the t(14;18) translocation, and are the more common subtype in cases arising from FL.
On the other hand, the activated B-cell (ABC) subtype derives from cells without ongoing somatic hypermutation. These lymphomas more commonly have constitutively activated nuclear factor κB (NF–κB) leading to overexpression of genes under the control of this transcription factor and often involve extranodal sites (e.g., testis, breast). These two subtypes of DLBCL have distinct survival differences (Alizadeh et al. 2000; Lenz et al. 2008; Rosenwald et al. 2002), with the GCB subtype having a better prognosis. Even with the incorporation of rituximab the ABC subtype is associated with an inferior prognosis. Subsequent investigations have proposed additional molecular signatures that appear to influence tumor behavior (Rosenwald et al. 2002; Lenz et al. 2008).
Gene expression profiling is not widely available as a clinical tool. Multiple immunohistochemical algorithms have been designed to predict whether a patient has a GCB or ABC subtype (Hans et al. 2004; Choi et al. 2009; Muris et al. 2006; Natkunam et al. 2008; Nyman et al. 2009; Meyer et al. 2011). No single algorithm is perfect in regards to predicting gene expression profiling results, though each divides patients into groups with different survival outcomes. While personalized therapy may ultimately be based on the molecular subtype of DLBCL, this has not currently been adequately examined.
MYC gene rearrangements are found in approximately 10 % of cases of DLBCL and portends an inferior prognosis (Barrans et al. 2010; Savage et al. 2009). When associated with a concurrent translocation t(14;18) involving BCL2, this is referred to as a double-hit DLBCL. The inferior prognosis is felt to be due to the concurrent expression of BCL2 and cMYC (Niitsu et al. 2009; Friedberg 2012). This phenomenon occurs in both GCB and ABC subtypes and retains independent prognostic importance even when the molecular subtype is taken into account (Klapper et al. 2008). Double-hit DLBCL occurs more frequently in the elderly.
3.3 Positron Emission Tomography
Similar to HL, PET has become an important imaging modality in the work-up and response assessment of DLBCL. In terms of the latter, both interim and post-chemotherapy PET assessment provide important prognostic information.
Post-chemotherapy PET response is a powerful prognostic factor, particularly when patients receive chemotherapy without consolidation radiation therapy. In numerous studies, residual PET positive disease after the completion of chemotherapy alone in aggressive NHLs (mostly DLBCL) is associated with a high risk of disease progression (Spaepen et al. 2001; Mikhaeel et al. 2000; Haioun et al. 2005; Juweid et al. 2005). For example, patients with advanced DLBCL with PET positive disease after chemotherapy had a 100 % risk of progression in the series by Mikhaeel et al. (2000) (n = 9) and Spaepen et al. (2001) (n = 26). Thus, observation without further assessment or adjuvant treatment is inappropriate when the PET remains positive after chemotherapy.
The risk of recurrence appears to be much smaller when consolidation radiation therapy is utilized in the setting of a persistently positive PET scan after chemotherapy. In studies from the Dana Farber Cancer Institute (Halasz et al. 2012) and Duke Cancer Institute (Dorth et al. 2011), mostly with early-stage disease, the risk of progression was 10 and 35 % respectively. While this progression risk appears to be higher compared with a negative PET, many patients remained disease free after radiation therapy. As gross residual disease is apparently present, the recommended dose is ~40 Gy.
Similar to HL, the role of interim PET in DLBCL is controversial. As outlined below, the positive predictive value of interim PET is ~50 %. Thus, many patients who have a positive interim PET will achieve a durable complete response after completion of therapy. Further, it is unclear that altering therapy simply because the PET remains positive after 2–3 cycles of chemotherapy improves outcomes. Finally, before embarking on a more aggressive therapy, particularly high-dose chemotherapy and autologous stem cell transplant, a biopsy to confirm persistent disease is generally recommended. This is often neglected in clinical practice. Despite these and other lingering questions, it is clear that interim PET response is prognostic and could potentially play a role in personalizing therapy.
Multiple studies have demonstrated that achievement of a negative PET early in the course of therapy is associated with improved progression-free survival, and often overall survival, compared with a positive interim PET (Zinzani et al. 2011; Cashen et al. 2011; Haioun et al. 2005; Yang et al. 2011; Safar et al. 2012). For example, in a study by Safar et al. (2012), 3-year progression-free and overall survival was 84 and 88 %, respectively, in patients achieving a negative PET after 2 cycles of chemotherapy plus rituximab. This compared with 47 and 62 %, respectively, in patients who were still PET positive. Interim PET response appears to have prognostic significance in both the low and high-risk IPI groups (Haioun et al. 2005; Yang et al. 2011). However, when compared with interim response assessment, post-chemotherapy PET response appears to be better, particularly in terms of the positive predictive value (Pregno et al. 2012; Cashen et al. 2011).
3.4 Treatment-Specific Factors
The addition of rituximab to combination chemotherapy has made the biggest impact on the overall prognosis of patients with DLBCL in the last several decades. On average, rituximab improves overall survival by ~10 % at 5 years (Feugier et al. 2005; Pfreundschuh et al. 2006). Rituximab improves clinical outcomes throughout the spectrum of disease presentations (early stage and advanced stage, younger age and older age, etc.).
Combination chemotherapy is currently considered the backbone of treatment for all stages of DLBCL, most commonly rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Yet, relapses after R-CHOP are common and most frequently involve sites of original involvement. Consolidation radiation therapy has been shown in many (Horning et al. 2004; Miller et al. 1998; Martinelli et al. 2009), but not all (Bonnet et al. 2007), randomized trials to decrease the risk of disease recurrence after CHOP in early-stage disease. Though no randomized trials have been completed in the rituximab era, RT also appears to be beneficial after R-CHOP (Phan et al. 2010). The role of radiation therapy in advanced DLBCL is controversial. Though infrequently employed, both prospective (Aviles et al. 1994, 2004) and retrospective (Ferreri et al. 2000; Schlembach et al. 2000; Dorth et al. 2012) studies have shown that consolidation radiation therapy decreases the overall risk of disease recurrence.
While adjuvant radiation therapy decreases the risk of relapse in patients with DLBCL, many are nonetheless successfully cured with chemotherapy alone and are unnecessarily exposed to the side effects, risks, and costs of treatment. This is not a unique phenomenon. In fact, it is a standard approach for all of oncology, in fact most of medicine, to treat a large number of patients to benefit a few. The challenge in DLBCL, which has thus far been elusive, is to identify those patients who cleared their systemic disease after chemotherapy yet still have residual microscopic local disease. A test to identify these patients has yet to be identified. Thus far, PET imaging after treatment has not been able to successfully identify a population of patients who do not benefit from radiation therapy (Dorth et al. 2012; Phan et al. 2010).
4 Follicular Lymphoma
4.1 Clinical Factors
Similar to the International Non-Hodgkin’s Lymphoma Prognostic Factors project for aggressive NHLs, a prognostic scoring system has also been developed for FL (Solal-Celigny et al. 2004). Using patient characteristics from 4,167 patients with FL diagnosed in the pre-rituximab era (1985–1992), five adverse prognostic factors for survival were identified on multivariate analysis. These included age, stage, hemoglobin level, number of nodal areas, and serum LDH. The hazard ratios ranged from 2.38 (age) to 1.39 (number of nodal sites) (Table 4). Three risk groups were identified: low risk (0-1 adverse factor), intermediate risk (2 factors), and poor risk (≥3 adverse factors). Ten-year overall survival was 71, 51, and 36 %, respectively (Fig. 4). This scoring system is known as the Follicular Lymphoma International Prognostic Index or FLIPI.
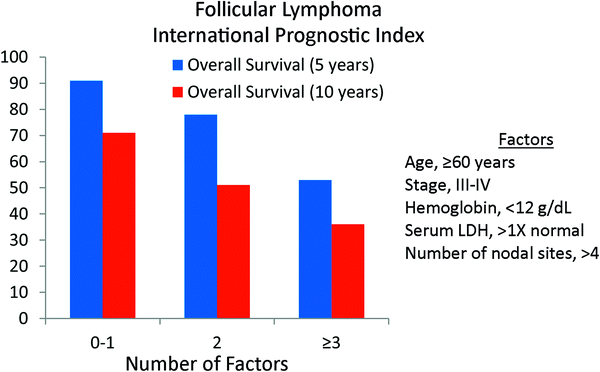
Table 4
Follicular Lymphoma International Prognostic Index (FLIPI)
Adverse prognostic factor | Relative risk |
|---|---|
Age, ≥60 years | 2.38 |
Stage, III-IV | 2.00 |
Hemoglobin (<12 g/dL) | 1.55 |
Serum LDH, >1X normal | 1.50 |
Number of nodal sites, >4 | 1.39 |

Fig. 4
The Follicular Lymphoma International Prognostic Index (FLIPI). The primary endpoint was overall survival (Blood 2004; 104;1258)
The FLIPI prognostic scoring system has limitations. It included patients treated before rituximab was available, though the original FLIPI appears to retain prognostic value even when ritixumab is utilized (Nooka et al. 2012). Further, the data were collected retrospectively and limited by missing data and limited available clinical characteristics. Therefore, a prospective study was launched in 2003 in which data from 1,093 patients from 69 institutions were collected (Federico et al. 2009). Using these data the FLIPI2 was created. As opposed to the original FLIPI, the primary endpoint was progression-free survival. The variables used in the model were β2-microglobulin (≤1X normal vs. >1X normal), tumor size (>6 cm versus ≤6 cm), bone marrow involvement, hemoglobin level (<12 g/dL versus ≥12 g/dL) and age (≥60 vs. <60 years). The 3-year progression-free survival was 91, 69, and 51 % for low (0 factors), intermediate (1–2 factors) and high (3–5 factors), respectively (Fig. 5).
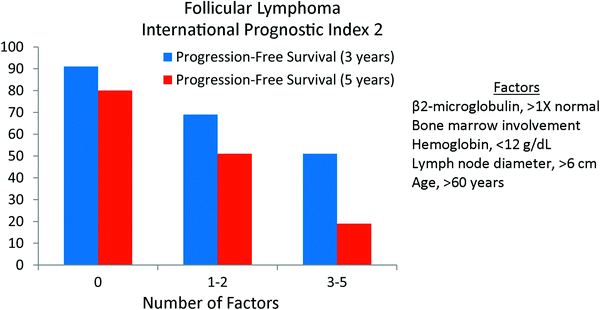

Fig. 5
The Follicular Lymphoma International Prognostic Index 2 (FLIPI2). The primary endpoint was progression-free survival (JCO 2009; 27:4555)
4.2 Pathological Factors
Follicular lymphoma is subdivided by the World Health Organization into three histological grades based on the number of centroblasts (large cells) in neoplastic follicles. Grades 1 and 2 are typically considered low grade and contain 0–5 and 6–15 centroblasts per high power field, respectively. Grade 3 FL is subdivided into 3A, where neoplastic follicles have more than 15 centroblasts per high power field but centrocytes (small cells) are still present, while 3B consists of solid sheets of centroblasts. If any diffuse areas are present this is considered DLBCL. Grade 3B FL is rare and most studies only have a small number of such patients, often preventing meaningful comparisons.
The prognostic significance of this grading system, and whether treatment should be modified based on grade, is controversial, with conflicting results in the literature. While some studies have demonstrated better outcomes with grade 1–2 FL compared with grade 3 (Ganti et al. 2006), others have not (Shustik et al. 2011; Chau et al. 2003). While grade 3B disease is often considered to be akin to DLBCL and treated as such, the literature is not consistent in this regard (Shustik et al. 2011; Chau et al. 2003; Ganti et al. 2006; Wahlin et al. 2012). Finally, some studies show an apparent plateau on the relapse-survival curve with anthracycline-based chemotherapy in grade 3 FL (Ganti et al. 2006; Wahlin et al. 2012) while others do not (Shustik et al. 2011; Chau et al. 2003). Thus, many questions remain whether the current grading system of FL can be used for prognostic purposes or to guide therapy.
Numerous other pathological factors have been investigated in FL including chromosomal alterations, overexpression of distinct genes as assessed by immunohistochemistry, genetics of the host, the microenvironment, transformation to high-grade disease, etc. (Relander et al. 2010). Currently, these factors are still poorly understood. How they should impact current management has not been elucidated. Validated prognostic indices, such as the FLIPI, combined with an assessment of disease presentation and clinical course by the oncologist remain the primary factors that guide therapy.
4.3 Positron Emission Tomography
The majority of patients with FL present with advanced disease. Advanced FL is generally considered an indolent disease, with a long disease course, but not curable with conventional chemotherapy. Patients with a low disease burden and who are asymptomatic do not require immediate treatment. For those requiring treatment, the most appropriate approach is combination chemotherapy with rituximab. Response to chemoimmunotherapy by PET imaging has been shown to be prognostic. In a prospective European study, patients with previously untreated, high-tumor burden FL received 6 cycles of R-CHOP and underwent interim and end of treatment PET imaging (Dupuis et al. 2012). Interim and post-treatment PET responses were closely correlated. Based on post-treatment PET imaging, 2-year progression-free survival was 87 % for PET positive patients vs. 51 % for PET negative patients, respectively (p = 0.005). There was also a difference in 2-year survival (100 vs 88 %, p = 0.13). Similar findings were observed in a subset of the PRIMA (Primary Rituximab and Maintenance) trial participants where post-treatment PET was predictive of progression-free and overall survival (Trotman et al. 2011).
4.4 Treatment-Specific Factors
Similar to DLBCL, the single most significant advance in the treatment of FL is the contribution of rituximab. Multiple randomized studies have shown that rituximab, added to a variety of different chemotherapy regimens, decreases the risk of progression and improves overall survival (Marcus et al. 2008; Hiddemann et al. 2005; Herold et al. 2007). Maintenance rituximab use, after a course of immunochemotherapy, further decreases the risk of relapse, but its effect on survival has not been conclusively demonstrated (van Oers et al. 2010; Salles et al. 2011). Thus, for patients with symptomatic or large burden advanced FL requiring treatment, a combination chemotherapy regimen combined with rituximab, with or without maintenance rituximab, is currently considered standard of care.
Only ~20 % of patients with FL present with localized disease. As opposed to advanced FL, there seems to be a potential for cure in this subgroup. The optimal treatment for patients with stage I, or localized stage II disease, is not clear. Most guidelines recommend involved-field radiation therapy. Long-term disease control is achieved in ~50 % of patients with low-doses (24–30 Gy) (Campbell et al. 2010; Guadagnolo et al. 2006; Mac Manus and Hoppe 1996; Vaughan Hudson et al. 1994). However, only ~33 % of patients with stage I disease are treated with radiation therapy (Friedberg et al. 2009; Pugh et al. 2010). A large study using Surveillance, Epidemiology, and End Results (SEER) data demonstrated superior survival in patients who received radiation therapy (62 vs. 48 % at 10 years, p < 0.001) (Pugh et al. 2010). However, results from the National LymphoCare Study have challenged the paradigm that radiation alone is optimal in stage I FL (Friedberg et al. 2012). With short follow-up, combined approaches using chemotherapy, immunotherapy, and radiation therapy were associated with a lower risk of relapse compared with radiation therapy alone with no difference in overall survival. The rarity of this stage of disease has precluded large phase III studies evaluating differing treatment approaches. With the understanding that neither chemotherapy nor immunotherapy is curative in FL, the minimal morbidity and cost of low-dose involved-field radiation therapy, which provides long-term disease control in a significant proportion of patients, suggests this is still the most appropriate treatment strategy for localized disease.
5 Non-Hodgkin Lymphomas: Other
5.1 Marginal Zone Lymphoma
Marginal zone lymphomas consist of three distinct entities- nodal marginal zone lymphoma, splenic marginal zone lymphoma, and extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT). The latter is most common and most extensively studied. MALT typically presents with localized disease most commonly involving the stomach, orbital adnexa, salivary glands, thyroid, or skin.
With the exception of Helicobacter pylori (H. pylori) positive gastric MALT lymphoma, first-line treatment for localized MALT lymphoma is typically radiation therapy. Complete response rates approach 100 % with doses ~25–30 Gy (Goda et al. 2010). The primary prognostic factor is disease site. MALT arising in paired organs (e.g., orbit or salivary gland) appears to have a higher risk of relapse compared with that arising in a single organ (e.g., stomach or thyroid) (Goda et al. 2010; Tsang et al. 2003). Relapse after radiation therapy for cutaneous marginal zone lymphoma is also rather frequent (Senff et al. 2007b). However, many relapses are localized, often in the contralateral paired organ, and after appropriate treatment, often further radiation therapy alone, long-term survival is excellent.
For patients with gastric MALT lymphoma, antibiotic treatment is indicated in the presence of H. pylori infection. Eradication of H. pylori is near universal after antibiotics. A complete response is achieved in approximately 80 % of patients, though this may take up to 2 years or longer to achieve (Wundisch et al. 2005). As long as the lymphoma is regressing on serial endoscopy, a watch and wait policy is appropriate. Predictive factors associated with a lower chance of achieving a complete response include deep gastric wall invasion (Nakamura et al. 2001; Sackmann et al. 1997; Ruskone-Fourmestraux et al. 2001), nodal involvement (Ono et al. 2010; Ruskone-Fourmestraux et al. 2001), and the translocation t(11;18) (Wundisch et al. 2005; Levy et al. 2005).
Of patients achieving a complete response, approximately 30 % of patients will relapse. The risk of relapse appears to be higher for those patients harboring the translocation t(11;18) and when ongoing B-cell monoclonality is detected by polymerase chain reaction (Wundisch et al. 2005). For patients who are H. pylori negative, do not respond to or relapse after antibiotics, the treatment of choice is radiation therapy. Long-term progression-free and overall survival exceeds 90 % (Goda et al. 2010). Interestingly, the original B-cell clone is also detected frequently after radiation therapy for gastric MALT lymphoma but without an associated risk of relapse (Noy et al. 2005).
5.2 Mantle Cell Lymphoma
Mantle cell lymphoma is a rare subtype of NHL. The majority of patients present with advanced disease. It is an aggressive lymphoma, typically arising in older adults, and not considered curable with conventional chemotherapy. The International Prognostic Index, utilized extensively for DLBCL, was not satisfactory in mantle cell lymphoma, which prompted development of a prognostic index specifically for this subtype of NHL.
The German Low Grade Lymphoma Study Group and the European Mantle Cell Lymphoma Network combined data to identify prognostic factors, apply the IPI and FLIPI, and design a specific model for mantle cell lymphoma. Using data from 455 patients with advanced disease entered on three consecutive randomized trials between 1996 and 2004, four independent adverse prognostic factors were identified (Hoster et al. 2008). These included increasing age, compromised performance status, elevated LDH, and elevated white blood cell count. Using a complex formula, a mantle cell lymphoma international prognostic index (MIPI) can be calculated with partitioning of patients into three risk groups. A simplified prognostic index (sMIPI) was also developed. Most studies have shown that the MIPI retains prognostic significance when applied to patients treated with modern rituximab-containing regimens with autologous stem cell transplantation (Budde et al. 2011; Geisler et al. 2010).
A small proportion of patients present with localized mantle cell lymphoma. The optimal treatment for this population is unclear. Based on general principles of lymphoma management, the inclusion of consolidation radiation therapy into the treatment program seems justified. Indeed, retrospective studies have shown favorable outcomes when radiation therapy is employed (Bernard et al. 2013; Leitch et al. 2003).
Finally, mantle cell lymphoma is a heterogeneous disease. Although most cases display aggressive behavior, there is a subset of patients with indolent disease. These patients occasionally present with slowly growing tumors that are amenable to low-dose radiation therapy. Mantle cell lymphoma can be exquisitely radiosensitive, and doses of ~10 Gy or even less often provide excellent palliation.
5.3 Systemic Anaplastic Large Cell Lymphoma
Systemic anaplastic large cell lymphoma (ALCL) is a relatively rare subtype of NHL that occurs in both children and adults. ALCL is a CD30 + T-cell neoplasm that often expresses the protein anaplastic lymphoma kinase (ALK), a protein that is not expressed normally in humans except occasionally in the brain. The most common chromosomal abnormality that leads to ALK expression is t(2;5) (p23; q35), though several others have been reported. The World Health Organization currently distinguishes ALK-positive ALCL as a distinct entity and ALK-negative ALCL as a provisional entity. ALK positive cases occur more commonly in the first three decades of life with ALK negative cases occurring predominantly in older adults.
Several studies have shown that survival is significantly better with ALK-positive ALCL (Sibon et al. 2012; Falini et al. 1999; Gascoyne et al. 1999; Savage et al. 2008). The International Peripheral T-cell Lymphoma Project demonstrated 5-year failure-free survival of 60 % for ALK-positive ALCL and 36 % for ALK-negative ALCL (p = 0.15). A difference in 5-year overall survival was also observed (70 vs 49 %, p = 0.16). There remains some uncertainty whether ALK expression is independently prognostic or simply correlates with other favorable factors (e.g., young age). A recent report from the Groupe D’Etude des Lymphomas de l’Adulte demonstrated only an elevated β2-microglobulin and older age as independent prognostic factors, but not ALK status (Sibon et al. 2012). ALK was also not independently prognostic in the study by Suzuki et al. (2000) However, other reports have demonstrated independent prognostic significance of ALK (Falini et al. 1999; Gascoyne et al. 1999).
5.4 Primary Central Nervous System Lymphoma
Primary central nervous system lymphoma (PCNSL) is a rare subtype of DLBCL that arises in the brain, spinal cord, and/or eyes. Whole brain radiation therapy (WBRT) was historically the treatment of choice with ~80 % of patients achieving a complete response. Unfortunately, relapses are common after WBRT alone with a median survival of ~12–18 months (Shibamoto et al. 2005). High-dose methotrexate regimens, with or without WBRT, have become the treatment of choice with an improvement in median survival to ~50–60 months in many studies (Abrey et al. 2000; Pels et al. 2003).
The contribution of WBRT after high-dose methotrexate regimens is controversial. In older patients (>60 years), conventional doses of radiation therapy (45 Gy) have been associated with unacceptably high rates of severe neurotoxicity, including dementia (Omuro et al. 2005), and should be avoided. Although conventional WBRT may decrease the risk of relapse, it is often counterbalanced by increased toxicity (Abrey et al. 2000). In the setting of a complete response with high-dose methotrexate, lower doses of WBRT may decrease the risk of relapse but avoid the toxicity of full-dose treatment (Shah et al. 2007). However, longer follow-up will be required to confirm these initial findings.
Though a number of prognostic factors have been identified in PCNSL (Corry et al. 1998; Bessell et al. 2004; Ferreri et al. 2003), the most important factors appear to be age and performance status. The Memorial Sloan-Kettering Cancer Center Prognostic Model, using recursive partitioning and external validation, identified three subgroups with distinct survival outcomes: class 1 (<50 years), class 2 (≥50 years, Karnofsky performance score [KPS] ≥ 70), and class 3 (≥50 years, KPS < 70) (Abrey et al. 2006). Median survival was 8.5, 3.2, and 1.1 years in classes 1–3, respectively.
5.5 Cutaneous Lymphomas
The cutaneous lymphomas present interesting diagnostic and therapeutic challenges given that they are relatively rare with an incidence of less than 5 per 1,000,000 individuals and present in a variety of different ways. Cutaneous lymphomas present with either a B-cell or T-cell phenotype. Mycosis fungoides (MF) is the most common of all of the cutaneous lymphomas. It is of the T-cell variety and typically presents clinically as patches and plaques on the skin but can involve lymph nodes and visceral organs. Another T-cell lymphoma that commonly involves the skin is anaplastic large cell lymphoma. This must be distinguished from the benign skin disorder lymphomatoid papulosis, another CD30 + lymphoproliferative disorder. Compared to its nodal counterpart discussed above, the cutaneous variant generally does not express the anaplastic lymphoma kinase (ALK). The B-cell lymphomas generally present differently than MF, and tend to be more isolated and often more nodular in appearance. The three most common types are: primary cutaneous diffuse large B-cell lymphoma, leg type, primary cutaneous marginal zone B-cell lymphoma, and primary cutaneous follicle center lymphoma (Senff et al. 2008; Girardi et al. 2004; Smith et al. 2004).
Mycosis fungoides is considered an indolent lymphoma and patients generally receive a variety of treatments during the course of their disease, including RT. With the exception of early, localized disease, treatment is not generally considered curative. Studies have evaluated numerous prognostic factors for different endpoints (overall survival, disease-specific survival, etc.). In terms of overall survival, the most consistent adverse prognostic factors include increasing age, higher T stage, presence of extracutaneous disease, elevated LDH and peripheral blood involvement (Kim et al. 1995, 2003; Diamandidou et al. 1999; Agar et al. 2010; Talpur et al. 2012). Folliculotropic histology (Agar et al. 2010) and large cell transformation also portends poor survival (Diamandidou et al. 1998). Primary cutaneous anaplastic large cell lymphoma, the other common T-cell cutaneous lymphoma, is much less common than MF and generally has a more favorable prognosis. Advancing age appears to be the most significant adverse prognostic factor (Liu et al. 2003; Woo et al. 2009). Progression to extracutaneous disease is also a likely adverse factor (Woo et al. 2009).
The prognosis of primary cutaneous marginal zone B-cell lymphoma and primary cutaneous follicle center lymphoma is excellent. For the majority with localized disease, RT alone produces complete response rates of nearly 100 % with excellent long-term survival (Senff et al. 2007b). Patients with marginal zone lymphoma may be at higher risk of relapse compared with follicle center lymphoma, though most recurrences are confined to the skin and typically amenable to a second course of RT (Senff et al. 2007b). On the other hand, primary cutaneous large B-cell lymphoma, leg type is associated with a much worse prognosis and a combined modality approach, consisting of chemotherapy and radiation therapy, is often employed (Senff et al. 2007a, b; Zinzani et al. 2006).
6 Toxicity
Long-term toxicity from RT is dependent on many variables. These include the type of lymphoma, radiation dose, normal structures within the radiation field, other treatments received (surgery and chemotherapy), age of the patient, and other medical co-morbidities. Unfortunately, many oncologists view risks of long-term toxicity from radiation therapy from a simplistic dichotomous viewpoint- either radiation was administered or it was not. This perception has contributed to the decline in the utilization of radiation therapy for many types of lymphomas. A more comprehensive assessment is necessary to weigh risks and benefits of treatment and to fully inform patients and other providers.
As lymphoma is a systemic disease that can affect any organ, it would be challenging to comprehensively address normal tissue toxicity. The two most troublesome complications after lymphoma treatment, particularly for HL, are secondary malignancies and cardiovascular disease. The most common complication is likely hypothyroidism. These will receive further discussion.
6.1 Secondary Malignancies
6.1.1 Hodgkin Versus Non-Hodgkin Lymphoma
Current survivors of HL are at increased risk of developing secondary malignancies. This is particularly true for patients treated in decades past with historical regimens such as subtotal nodal irradiation and chemotherapy regimens incorporating high doses of alkylating agents. In large registries of patients treated over many decades, second cancer risk has consistently been elevated over expected rates. Using data from 32,591 HL patients from 16 North American and European cancer registries, treated between 1935–1994, the observed-to-expected ratio was 2.3 (95 % CI 2.2–2.4). A similar study of 10,472 survivors from North America between 1940–1987 demonstrated an observed/expected ratio of 2.7 (95 % CI 2.5–4.2) (Boivin et al. 1995). Finally, a standardized incidence ratio of 2.9 (95 % CI 2.6–3.2) was noted in a cohort of 5519 British patients treated between 1963–1993 (Swerdlow et al. 2000). It should be remembered that during these time periods definitive subtotal nodal irradiation was standard and many patients also received alkylating agents.
The risk of developing a secondary malignancy after treatment for NHL has not been as extensively studied compared with HL and is therefore less understood. In an early study from Duke Cancer Institute, among 686 patients with non-Hodgkin lymphoma the risk of developing a solid tumor was similar to the general population. The risk of leukemia was elevated, though the incorporation of radiation therapy did not increase risk (Lavey et al. 1990).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree