CHAPTER 53 Magnetic Resonance and Computed Tomographic Imaging of Myocardial Perfusion
Data from the National Health and Nutrition Examination Survey (1999-2004, National Heart, Lung and Blood Institute) estimate the prevalence of coronary heart disease to be 16 million individuals in the United States, and incidence of new and recurrent coronary events to be 1.2 million per year.1 Data from 44 years of follow-up in the original Framingham Heart Study cohort and 20 years of their offspring surveillance show the lifetime risk of developing coronary heart disease for individuals 40 years old to be 49% in men and 32% in women.2 With mortality statistics claiming that one out of every five deaths is the result of coronary artery disease (CAD),1 there is a clear need for improved diagnostic imaging strategies for detecting coronary heart disease and myocardium vulnerable to scenarios of reduced perfusion.
The clinical presentation of ischemic heart disease is incredibly diverse and includes asymptomatic or silent ischemia, chronic angina, unstable angina or infarction (i.e., ACS), new-onset or chronic heart failure, or sudden cardiac death. Although symptomatic patients are often identified by symptoms related to angina or heart failure, patients who have silent ischemia represent a diagnostic dilemma because they are less likely to be referred for testing. Patients who are particularly susceptible to silent ischemia include diabetics, elderly patients, and patients with prior MI or surgical revascularization.3
Noninvasive imaging is a crucial component in the evaluation of patients with suspected ischemic heart disease. Figure 53-1 illustrates an algorithm for the management of patients with potential ischemic heart disease. For patients presenting with a possible ACS who have a negative ECG and biomarkers (e.g., troponin), a stress test can be used to identify high-risk patients who would benefit from being admitted to the hospital for further evaluation and testing. The American College of Cardiology/American Heart Association guidelines for the management of unstable angina/non–ST segment elevation MI suggest that coronary CT angiography is a reasonable alternative to stress testing in patients with low to intermediate probability of CAD in whom initial ECG and initial biomarkers are unremarkable.4
Patients who are admitted with an ACS can be treated with an early invasive or early conservative strategy. High-risk patients have been shown to benefit from early interventions, and warrant early referral for invasive angiography. For low-risk patients, and in particular women, a conservative strategy that uses noninvasive testing is recommended.4
For patients with chronic stable angina, the American College of Cardiology/American Heart Association guidelines5 suggest that ejection fraction should be measured for all patients with a history of MI or signs suggesting heart failure. In the presence of a systolic murmur suggestive of aortic stenosis, mitral regurgitation, or hypertrophic cardiomyopathy, an echocardiogram should be obtained.
As is shown in Figure 53-1, all patients with chronic angina (i.e., stable angina) should have an evaluation for the presence of ischemic heart disease. This evaluation can be accomplished by ECG exercise testing, noninvasive stress imaging, or invasive angiography. Higher risk patients or patients with abnormal baseline ECG should be referred for an imaging-based test. The goal of such a test is to identify the extent, severity, and location of ischemia and when possible provide information about prognosis.
The role of cardiac imaging for the detection of silent ischemia is controversial. Patients with diabetes may benefit from such a strategy because they have a high risk of cardiovascular-related mortality, are more likely to have silent ischemia, and are less likely to survive an MI than nondiabetic patients.6 Nevertheless, the cost-effectiveness of screening all such at-risk patients with nuclear perfusion imaging is controversial.7
This chapter focuses on the evaluation of MRI and CT for the assessment of myocardial perfusion (Figs. 53-2 and 53-3). The advantages and disadvantages of other imaging modalities are discussed and compared with MRI and CT, and these imaging modalities are put in clinical perspective with descriptions of how and when they can facilitate the diagnosis and management of patients suspected to have CAD or with known CAD.
MAGNETIC RESONANCE IMAGING
Preclinical and Clinical Evaluation
Numerous animal studies have shown good correlation of MRI perfusion with tissue perfusion as measured by radioactive microspheres.8,9 Notable studies included work by Wilke and colleagues and Klocke and associates,8 in which porcine and canine models of left circumflex coronary artery stenosis were created that showed that MRI can detect different degrees of myocardial perfusion under adenosine stress.
Subsequently, Lee and colleagues9 used a canine model of left circumflex coronary artery stenosis and compared perfusion MRI with Tc 99m sestamibi and thallium 201. They showed that MRI could detect perfusion defects with a left circumflex coronary artery stenosis of 50% or greater, whereas SPECT perfusion defects were detected only with left circumflex coronary artery stenosis of 85% or greater.
Multiple clinical human studies testing the diagnostic accuracy and performance of stress perfusion MRI were performed comparing MRI with nuclear imaging and conventional coronary angiography. Table 53-1 summarizes published data with x-ray angiography as the reference standard. Nandalur and associates10 performed a meta-analysis of all stress MRI studies with two main techniques in use, perfusion imaging and imaging of stress-induced wall motion abnormalities, from January 1990 to January 2007 with a total of 37 studies involving 2191 patients. All studies used catheter x-ray angiography as the reference standard. Fourteen studies (N = 754 patients) using stress-induced wall motion abnormalities imaging showed 83% sensitivity and 86% specificity on a patient level. Perfusion imaging showed a sensitivity of 91% and specificity of 81% on a patient level (disease prevalence 57.4%).
TABLE 53-1 Diagnostic Accuracy of Stress Perfusion Magnetic Resonance Imaging Studies, Having Invasive Coronary Angiography as the Reference Standard
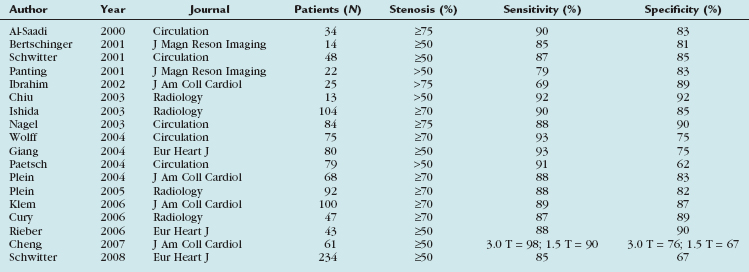
Two more recent studies by Cury and associates11 and Klem and coworkers12 (see Table 53-1) sought to improve the diagnostic accuracy of stress perfusion MRI by using a comprehensive imaging approach of first-pass contrast administration for myocardial perfusion at rest and stress with the addition of myocardial delayed enhancement (MDE) imaging for infarct detection and characterization. Our group showed that this combined approach has 87% sensitivity, 89% specificity, and 88% accuracy, and was superior to rest/stress perfusion MRI alone, which has 81% sensitivity, 87% specificity, and 85% accuracy, having invasive coronary angiography as the reference standard. Klem and coworkers12 showed similar results—that stress perfusion and MDE MRI can better detect inducible ischemia and fixed defects compared with stress/rest perfusion MRI. MDE MRI also provides the benefit of improved identification of regions of no reflow or microvascular obstruction, which is readily identified on MDE as regions of no hyperenhancement within the hyperenhancing infarction (see Figs. 53-2 and 53-3).
In another study evaluating patients presenting to the emergency department with acute chest pain, Ingkanisorn and colleagues13 evaluated the diagnostic value of adenosine stress myocardial perfusion MRI in 135 patients who presented to the emergency department with chest pain and a negative initial troponin value. The main study outcome was the detection of any evidence of significant CAD. Patients were contacted at 1 year to determine the incidence of significant CAD, defined as significant coronary artery stenosis (>50%) on invasive coronary angiography, abnormal correlative stress test, new MI, or death. Adenosine myocardial perfusion MRI abnormalities had 100% sensitivity and 93% specificity for detection of significant CAD, and an abnormal MRI added significant prognostic value in predicting a future diagnosis of CAD, MI, or death over clinical risk factors. No patients with a normal adenosine myocardial perfusion MRI had a subsequent diagnosis of CAD or an adverse outcome.
The first multicenter trial involving 18 centers in Europe and the United States (MR-IMPACT trial) comparing stress myocardial perfusion MRI with SPECT imaging and invasive coronary angiography was published more recently.14 When comparing perfusion MRI at 0.1 mmol/kg versus the entire SPECT population, the receiver operating curve analysis showed a better performance for perfusion MRI (n
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


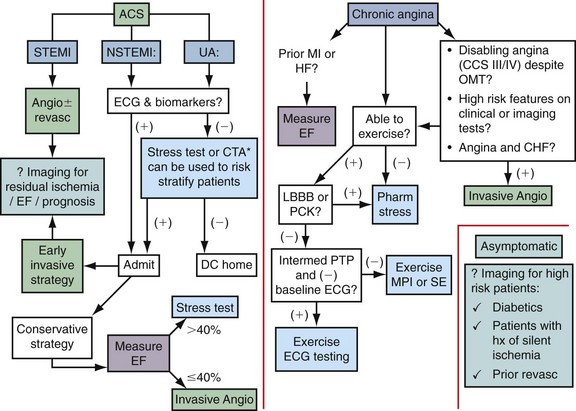
 FIGURE 53-1
FIGURE 53-1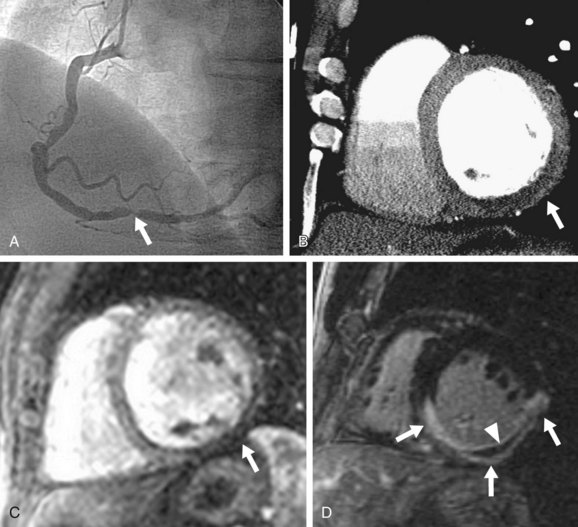
 FIGURE 53-2
FIGURE 53-2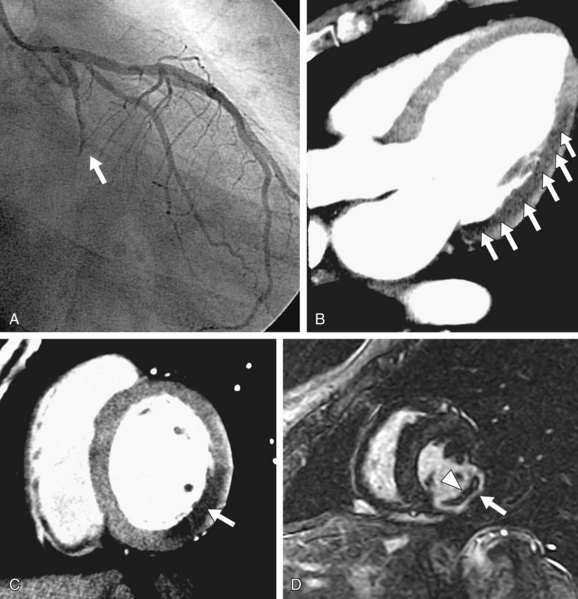
 FIGURE 53-3
FIGURE 53-3