Fig. 8.1
Soft pillow driver used to introduce shear waves into the brain positioned within a standard eight channel head coil
Pulse Sequence
Brain MRE data is typically acquired using a modified single-shot, spin-echo EPI pulse sequence. Shear waves of 60 Hz are introduced using the pneumatic active driver (located outside of the scanner room) and a soft, pillow-like passive driver placed under the subject’s head as previously described [6]. The resulting motion is imaged with the following parameters: TR/TE = 3,600/62 ms; field of view (FOV) = 24 cm; BW = ±250 kHz; 72 × 72 imaging matrix reconstructed to 80 × 80; frequency encoding in the right-left direction; 3x ASSET (SENSE) acceleration; 48 contiguous 3 mm thick axial slices; one 4 G/cm, 18.2 ms, zeroth- and first-order moment nulled motion encoding gradient on each side of the refocusing RF pulse synchronized to the motion; motion encoding in the positive and negative x, y and z directions; and eight phase offsets sampled over one period of the 60 Hz motion. The resulting images have 3 mm isotropic resolution and are acquired in less than 7 min.
Meningioma
The application of brain MRE to focal tumors has been proposed [12] and has already been found useful for evaluating meningiomas prior to resection [13]. Most meningiomas are characterized on MRI imaging by isointense T1 and T2 signal with uniform gadolinium contrast enhancement. However, despite their frequent uniform appearance, their viscoelastic properties range widely from very soft and removable by suction to very stiff requiring extensive dissection including the use of ultrasonic aspirators. While it is known that extremely soft meningiomas are seen on MRI as increased T2 signal with decreased T1 signal, and that extremely stiff meningiomas present with decreased T2 signal, the physical characteristics of the vast majority of meningiomas are unknown prior to surgery [14]. This fact impacts clinical care because extremely stiff meningiomas, especially those located at the skull base, are associated with increased surgical risk due to the potential for damage to adjacent and encompassed arteries, veins, and nerves. Various techniques have been attempted to preoperatively identify stiffness including standard MRI [14, 15], MR spectroscopy [16], MR diffusion imaging [17], and CT [18].
MRE has been shown to be a good preoperative assessment tool for meningioma properties, improving both risk assessment and patient management [13]. A series of meningiomas were evaluated using a five grade scale, ranging from soft and 100 % removable with suction to uniformly hard, requiring that most of the tumor be removed with ultrasonic aspiration (Fig. 8.2). Meningioma stiffness measured by MRE compared to the surgeons’ qualitative assessment of tumor stiffness determined at the time of surgery showed stiffness significantly correlated with the qualitative assessment at surgery (p = 0.023) [13].
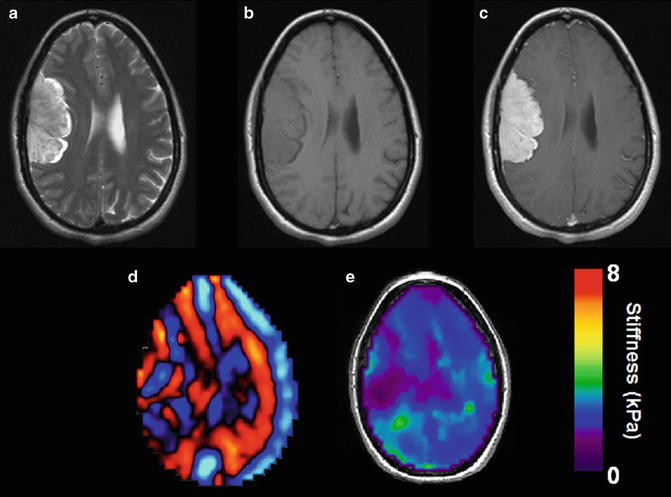

Fig. 8.2
Approximate 6 × 3.5 cm dural based extraaxial tumor with mildly increased T2 signal (a) and isointense T1 signal (b). Relatively uniform contrast enhancement following the administration of gadolinium contrast (c). Wave image (d) and the elastogram (e) show the meningioma is softer than surrounding brain tissue. This was confirmed at surgery where the lesion was easily resected with suction
The introduction of high resolution MRE with 3 mm isotropic spatial resolution has allowed the identification of internal heterogeneity within meningiomas (Fig. 8.3). As meningiomas enlarge the internal tumor components may develop variable regions of stiffness. Often calcification either on the tumor surface or internally leads to considerable stiffening. Also, the density of the fibrotic components of tumor without frank calcification may lead to very stiff tissue. Internal necrosis due to ischemic or hemorrhagic change may result in regional softening (Fig. 8.3). To provide the surgeon with a preoperative map of the variable internal tissue characteristics may lead to altered surgical plans with a safer potentially more complete resection of the lesion.
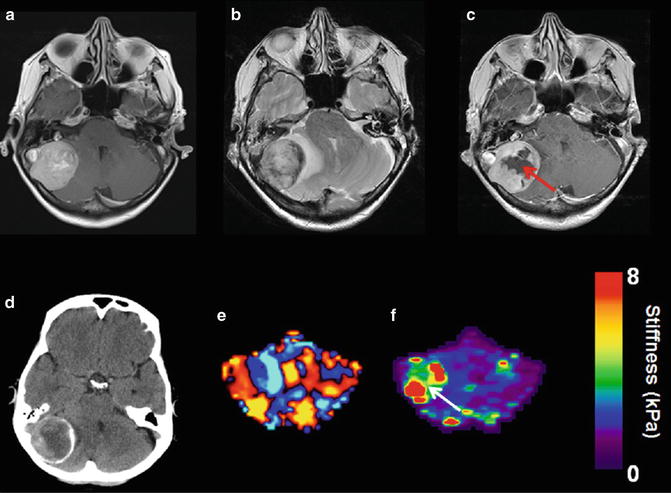

Fig. 8.3
Example of an approximate 4 cm right posterior fossa meningioma with varied internal viscoelastic characteristics. MRI T1 weighted post gadolinium image from 2011 demonstrates uniform enhancement throughout the lesion (a). T2 weighted image from a followup 2014 exam shows the interval development of surrounding vasogenic edema and increased mass effect (b). T1 weighted post gadolinium image shows central loss of contrast enhancement that corresponded to histological changes of hemorrhagic necrosis (arrow c). CT demonstrated peripheral calcification (d). Wave image shows the shear wavelength is longer in this stiff meningioma (e). Elastogram shows heterogeneity corresponding to the very stiff peripheral tumor and soft central necrosis (arrow f)
Glioma
Gliomas are the most frequent type of primary tumors arising in the brain. A biopsy is required for accurate pathologic characterization of the tumor grade and is based on the World Health Organization grading system [19]. The most common type of gliomas are astrocytomas which are classified as pilocytic astrocytoma (grade I), low-grade astrocytoma (grade II), anaplastic astrocytoma (grade III), and glioblastoma multiforme (grade IV). While standard imaging findings can be suggestive such as the presence of contrast enhancement and necrosis, no non-invasive technique exists for the preoperative classification of glioma tumor grade [20]. The potential exists that the addition of stiffness information may allow improved preoperative characterization of glioma subtypes (Fig. 8.4).
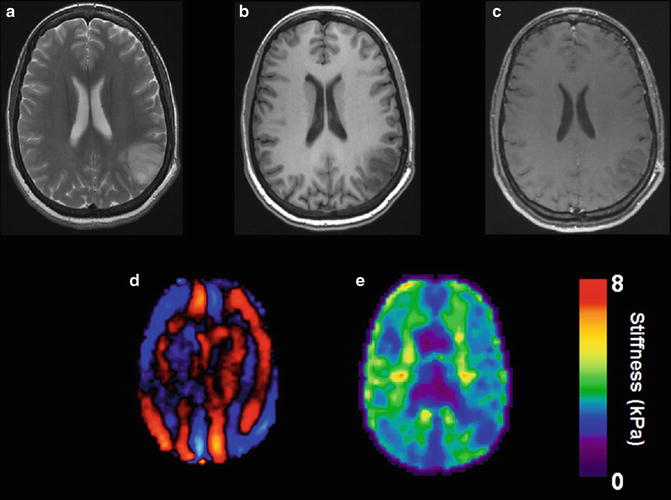

Fig. 8.4
Example of an oligodendroglioma in a 34 year old female discovered following a single seizure. T2 weighted image demonstrates an infiltrative lesion with involvement of both the white matter and overlying cortex with enlargement of the gyri typical of oligodendroglioma (a). Low T1 signal is seen before the administration of gadolinium (b) with no definite enhancement demonstrated (c). Wave image (d) and elastogram (e) shows the tumor to be softer than surrounding normal brain tissue
The possibility of utilizing MRE as a biomarker to determine cancer therapy response has already been demonstrated in non-Hodgkin’s lymphoma and colon cancer [21, 22]. Following chemotherapy, MRE measured a significant decrease in tumor stiffness before a clinically significant change in tumor volume, suggesting MRE may offer an earlier indication of treatment response compared with current clinical methods. Changes in the mechanical properties of the extracellular matrix have been shown to influence cancer progression [23], where stiffening of the matrix promotes the spread of malignant cells. MRE is capable of quantifying change in the tissue mechanical properties, and may be able to identify increased brain tissue stiffness surrounding the tumor that may indicate the spread of disease. A noninvasive assessment of tumor spread and a prediction of recurrence would have a significant impact on patient care.
Pituitary Adenoma
Pituitary adenomas are a common tumor of pituitary gland that may result in abnormally increased hormone production. Some adenomas are not associated with endocrine changes and as a result may reach large size before indirect symptoms lead to their discovery. When possible transsphenoidal is the preferred surgerical approach for resection of the tumor [24]. Surgical risk increases with increased size, lobulation, invasion of the adjacent cavernous sinuses, and increased stiffness of tumor [25]. Soft lesions are often removed with suction through a transsphenoidal approach. However, stiff lesions can be very difficult to remove in large part due to the limited surgical operating field of view and therefore an open craniotomy is preferred. MRE offers a preoperative evaluation of tumor stiffness and improved surgical planning for the safest resection technique (Fig. 8.5).
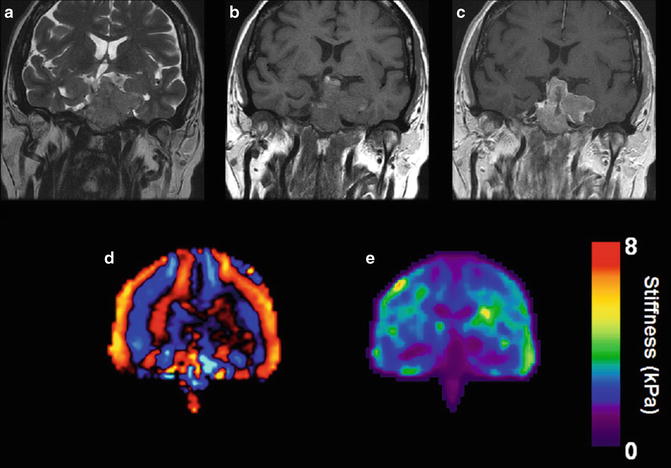

Fig. 8.5
Very large sellar mass invading the left cavernous sinus and clivus extending into and filling the sphenoid sinus which was demonstrated to represent a null cell pituitary adenoma. Coronal imaging shows the tumor to be essentially isointense to brain tissue on T2 weighted (a) and T1 weighed (b) sequences. Mildly irregular enhancement is seen following the administration of gadolinium (c). Wave image (d) and elastogram (e) shows the tumor to be softer than brain tissue which was confirmed when the tumor was removed
Demyelination
MRI plays a prominent role in the diagnosis and management of demyelinating diseases such as multiple sclerosis (MS). MS is an immune mediated disease in which monocytes and T-cells induce an inflammatory response that causes both demyelination and degeneration of axons and gliosis. As the body’s immune cells damage the myelin, axons lose the ability to conduct electrical signals resulting in functional impairment. Recent studies demonstrate that the brain tissue involved in MS is not confined to the white matter, and there is a growing understanding of the role of cortical involvement [26]. MS is thus considered a diffuse disease of the central nervous system. Neuroimaging has come to be a standard tool for diagnosing MS. Gadolinium enhancement, the result of a breakdown of the blood–brain-barrier, is one of the earliest imaging findings and typically indicates active disease. The McDonald criterion, introduced in 2001 for the diagnosis of MS, gave MRI a major role [27]. MRI also has been used as a surrogate for clinical trials of new treatments. However, standard MRI findings are not always strongly correlated with disease severity or predictive of future relapse [28], a situation referred to as the “clinicoradiologic paradox.” To overcome this conundrum, a technique such as MRE that evaluates the structural integrity of brain tissue may have a role by characterizing tissue alterations not detected by standard imaging.
Wuerfel reported the results of an MRE study in which four different driver frequencies were used in 45 patients with mild relapsing-remitting MS. The study was conducted with a 1.5T scanner with a single-shot spin-echo EPI sequence [7]. They found that at 62.5 Hz, there was a 13 % decrease in the viscoelasticity in MS patients compared to matched healthy controls. This decreased stiffness was independent of standard MRI findings such as volume of T2 signal abnormality or enhancement. A follow-up study was conducted on 23 patients, focusing on chronic-progressive MS and utilizing similar techniques [29




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree