The advent of focal therapies theoretically offers new treatment options for patients with localized prostate cancer. The goal of prostate cancer treatment is effective long-term cure with minimal impact on health-related quality of life. Multiparametric MR imaging of the prostate is being increasingly used for diagnosis, image-guided targeted biopsy, guidance for targeted focal and regional therapy, and monitoring the effectiveness of treatments for prostate cancer of all stages. In this article, the use of prostate MRI in the burgeoning domain of thermal ablative therapy for localized and recurrent prostate cancer is reviewed.
Key points
- •
Whole-gland and focal MR imaging–guided thermal ablative treatments for native and recurrent prostate cancer include cryoablation, laser, and focused ultrasonography ablation.
- •
Integrated clinical and imaging workup for the native and recurrent prostate cancer should include optimal multiparametric MR imaging of the prostate, careful mapping/targeted biopsy, and judicial selection of patients with appropriate cross-sectional imaging of the body to assess regional and distant disease.
- •
Multicenter, prospective clinical trials are critically needed to assess thermal ablative treatment efficacy for native and recurrent prostate cancer.
The state of therapies for prostate cancer
The American Cancer Society estimates that 220,800 new cases of prostate cancer will be diagnosed in the United States in 2015. Prostate cancer is the most commonly diagnosed cancer in men. With an estimated 27,540 deaths in 2015, prostate cancer is the second-leading cause of cancer death in men. Many men with prostate cancer are often managed with radiotherapy, surgery, or androgen deprivation. No matter how expertly performed, these therapies carry significant risk and morbidity to the patient’s health-related quality of life, with potential impact on sexual, urinary, and bowel function. Active screening programs for prostate cancer have identified increasing numbers of low-risk prostate cancer and have encouraged regimens of active surveillance to delay treatment until cancer progression. Although active debate continues on the suitability of focal or regional therapy for these patients with low-risk prostate cancer, many unresolved issues remain, complicating this management approach, including prostate cancer multifocality, limitations of current biopsy strategies, suboptimal staging by accepted imaging modalities, and less than robust prediction models for indolent prostate cancers. Despite these restrictions, focal therapy continues to confront the current paradigm of therapy for low-risk disease. Furthermore, prostate cancer recurrence rates after established forms of therapy range from 20% to 60%. Advanced, locally recurrent, or metastatic disease has also become more amenable to treatment with new classes of medications and robotic surgical approaches. With such disease volume, the opportunities for treating advancement in early, recurrent, and metastatic disease are almost boundless. In this article, the use of MR imaging to direct focal therapy for native and recurrent prostate cancer is described.
The state of therapies for prostate cancer
The American Cancer Society estimates that 220,800 new cases of prostate cancer will be diagnosed in the United States in 2015. Prostate cancer is the most commonly diagnosed cancer in men. With an estimated 27,540 deaths in 2015, prostate cancer is the second-leading cause of cancer death in men. Many men with prostate cancer are often managed with radiotherapy, surgery, or androgen deprivation. No matter how expertly performed, these therapies carry significant risk and morbidity to the patient’s health-related quality of life, with potential impact on sexual, urinary, and bowel function. Active screening programs for prostate cancer have identified increasing numbers of low-risk prostate cancer and have encouraged regimens of active surveillance to delay treatment until cancer progression. Although active debate continues on the suitability of focal or regional therapy for these patients with low-risk prostate cancer, many unresolved issues remain, complicating this management approach, including prostate cancer multifocality, limitations of current biopsy strategies, suboptimal staging by accepted imaging modalities, and less than robust prediction models for indolent prostate cancers. Despite these restrictions, focal therapy continues to confront the current paradigm of therapy for low-risk disease. Furthermore, prostate cancer recurrence rates after established forms of therapy range from 20% to 60%. Advanced, locally recurrent, or metastatic disease has also become more amenable to treatment with new classes of medications and robotic surgical approaches. With such disease volume, the opportunities for treating advancement in early, recurrent, and metastatic disease are almost boundless. In this article, the use of MR imaging to direct focal therapy for native and recurrent prostate cancer is described.
Importance of MR imaging for prostate cancer imaging
Prostate cancer has traditionally been diagnosed by systematic but random sampling of the entire organ. The recent introduction of multiparametric MR imaging (mpMR imaging) now allows for imaging-based identification of prostate cancer, which may improve diagnostic accuracy for higher-risk tumors. Recently, a consensus panel agreed to PI-RADS v2 (Prostate Imaging-Reporting and Data System), which is designed to improve detection, localization, characterization, and risk stratification in patients with suspected cancer in treatment-naive prostate glands. Targeted biopsy of suspected cancer lesions detected by MR imaging is associated with increased detection of high-risk prostate cancer and decreased detection of low-risk prostate cancer, particularly with the aid of MR imaging/ultrasonography (US) fusion platforms. The use of mpMR imaging has expanded beyond staging to detection, characterization, and monitoring for active surveillance for cases of suspected recurrence. The use of MR imaging for recurrent prostate cancer continues to evolve and has potential to evaluate both local recurrence and distant bony and nodal metastases. In 2013, a consensus panel chaired by Professor Michael Marberger endorsed using mpMR imaging to identify patients for focal therapy. Multiparametric MR imaging is capable of localizing small tumors for focal therapy and is the technique of choice for follow-up of focal ablation. Although mpMR imaging plays an established, critical role in native and recurrent prostate cancer imaging, functional, metabolic imaging for prostate cancer is in its formative years. [ 11 C]Choline PET/computed tomography (CT) has an advantage in showing both local recurrent and distant metastatic prostate cancers. [ 11 C]Choline PET/CT had a sensitivity of 73%, a specificity of 88%, a positive predictive value of 92%, a negative predictive value of 61%, and an accuracy of 78% for the detection of clinically suspected recurrent prostate cancer in postsurgical patients. In a study of postprostatectomy patients with increasing prostate-specific antigen (PSA) levels, mpMR imaging was superior for the detection of local recurrence, [ 11 C]choline PET/CT superior for pelvic nodal metastasis, and both are equally excellent for pelvic bone metastasis. [ 11 C]Choline PET/CT and mpMR imaging are complementary for restaging prostatectomy patients with suspected recurrent disease. However, [ 11 C]choline PET/CT is not widely available.
With the limitations of US and PET/CT imaging, MR imaging remains preeminent for detection and staging of recurrent prostate tumors. MR imaging provides superior soft tissue contrast resolution, high spatial resolution, multiplanar imaging capabilities, and a large field of view.
If the focal treatment is intended for potential curative treatment, it is important to ensure that there is not distant disease with whole-body CT/MR imaging, bone scan, and [ 11 C]choline PET/CT. None of these imaging modalities is perfect, and appropriate selection of image staging is unique to each patient.
Native Prostate Cancer
In selecting the appropriate patient for focal therapy for the native prostate gland, it is critical to determine that the patient has localized low-risk disease. With low-risk disease, there is level 1 evidence that implies a lack of benefit from radical therapy. Patients are often targeted for cancer workup because of increasing PSA levels or nodule on digital rectal examination. Patients are further evaluated with a mapping biopsy or mpMR imaging with targeted biopsy. Patients are classified to have low or intermediate prostate cancer with a focal positive lesion on mpMR imaging, Gleason score 4 + 3 or lower, and PSA level less than 20 ng/mL. For consideration for focal therapy, the target lesion should be confined to 1 lobe of the prostate. Furthermore, the target should be visible with the imaging modality that will be used to guide the focal ablation treatment.
Focal Therapy Treatments for Native Prostate Cancer
Although radical prostatectomy and radiation therapy remain the preferred definitive therapy for choice for men with newly diagnosed prostate cancer and with a life expectancy greater than 10 years, there is increasing interest in less radical focal methodologies for treatment, especially in the watchful waiting population. For this population of patients with low-risk and intermediate-risk prostate cancer, active surveillance may be undesirable, yet the complications and comorbidities associated with standard therapies are still not palatable. This patient-driven interest is pushing the development of minimally invasive focal therapies for prostate carcinoma in low-risk patients. As a result, several minimally invasive thermal ablation methods under direct MR guidance, most prominently cryotherapy, laser ablation, and high-intensity focused US (HIFU), have been developed and are being evaluated.
Magnetic resonance–guided cryoablation
MR-guided percutaneous cryoablation offers the combination of superb soft tissue resolution typical of MR imaging coupled with the ablative capacity of cryosurgery. Early experience combining cryoablation with MR imaging has shown a high degree of accuracy in defining normal and frozen tissue on all MR imaging sequences. In addition, MR imaging allows visualization of the iceball in multiple planes, which becomes critically important in the pelvis, where there is limited safety for nontarget iceball growth. These features allow for more precise cryoprobe placement and iceball monitoring during treatment within the confined space of the pelvis. The final advantage is that this procedure is not appreciably limited by previous surgery or radiation to the treated area.
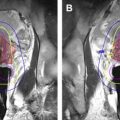
There are limited data using MR-guided cryoablation within the native prostate. Two published canine studies reported feasibility and overall safety. These studies did bring out 1 limitation of cryoablation, which is that the visualized edge of the ice (°C) does not represent the ablation margin. The ablation margin is best shown with contrast enhancement after the procedure and is at the –2°C isotherm, which is just inside the edge of the iceball. There are 2 published reports of MR-guided cryoablation in native prostate glands, each with small numbers ( Fig. 1 ). Gangi and colleagues performed MR imaging–guided prostate cryoablation in 11 patients using 1.5-T MR imaging. They had some minor complications of hematuria, dysuria, and urine retention. In addition, they had 1 major complication of rectal fistula, with spontaneous closure after 3 months. The other study examined 18 patients with 2 slightly different methodologies. The group treated with a more aggressive freezing regimen had better results over time. These studies confirm that MR-guided cryoablation is technically feasible with relative safety; however, more short-term and long-term data are needed to assess overall efficacy.
Magnetic resonance–guided laser ablation
Laser-induced interstitial thermal therapy (LITT) uses a locally placed laser fiber to deliver targeted thermal ablation. LITT is inherently MR compatible, making it an obvious choice of ablation technologies to couple with MR imaging. MR-based temperature monitoring allows real-time feedback during LITT treatment, because both deposition of light energy and MR signal acquisition can be performed simultaneously without degradation in the MR image. Performance of the ablation within the MR imaging allows use of posttreatment imaging to verify treatment delivery. Because MR images clearly show the prostate anatomy and the surrounding critical structures, MR imaging is critical for monitoring ablation growth to prevent encroachment onto adjacent critical structures.
Examination of the literature shows that LITT began being used in the brain to treat epileptogenic foci and neoplasms and expanded to liver lesions, in which its use is widely accepted. However, use of percutaneous laser ablation therapy in the prostate is relatively new. Two studies reported feasibility in canine prostate as well. These studies were beneficial because they showed technical feasibility but also correlation of the MR temperature map with contrast-enhanced T1-weighted images. A subsequent study in cadavers reported technical feasibility in the human prostate within a 3-T MR imaging scanner. Lee and colleagues published early results on 23 patients treated with focal laser ablation, showing promising results. Raz and colleagues described using laser ablation for treatment of 2 patients with prostate cancer at 1.5 T, with discharge 3 hours after the procedure. These studies show the potential usefulness of laser ablation in the prostate. However, more data are needed to determine short-term and long-term effects.
Magnetic resonance–guided focused US ablation
Treatment of the prostate with focused US ablation is not new, although an MR imaging– guided version of the procedure has not been approved by the US Food and Drug Administration (FDA) in the United States. It has been performed with transrectal US imaging guidance with success in Europe for many years. However, a major limitation of US imaging guidance is the difficulty in visualizing the focus of cancer, especially if the focus is small. Therefore, the treatment strategy used with US-guided HIFU is to ablate the entire prostate, or a relatively large region if the site of biopsy-proven cancer was found using a mapping biopsy or mpMR imaging. This procedure often results in inadequate tumor control or overablation of unnecessary normal/neural tissue, with potential subsequent morbidity. An early study by Gelet and colleagues used US-guided focused US ablation in the prostate of 82 patients who were treated and followed up for 24 months. These patients also received subsequent radiation treatment. Of the patients, 68% were cancer free at the time of follow-up. Because of relatively high complication rates, the treatment device underwent multiple iterations and improvements. A subsequent study by Gelet and colleagues reported incontinence and impotency rates of approximately 14% and 61%, respectively, at 19 months after treatment. In both studies, major limitations were identified as total procedure time as a result of need to cover the entire prostate and inability to monitor temperatures or ablation zone expansion. MR thermal monitoring and localization of lesions/zones within the prostate should allow optimization of an ablation treatment zone, whereas ablation temperature monitoring should allow an improved safety margin regarding vital adjacent tissues. There are 2 MR imaging integrated systems using transrectal or transurethral transmission routes for treatment of prostate lesions with focused US technology. The systems are fully integrated with the MR imaging console with temperature feedback control to adjust power, frequency, and rotation rate. Both systems are being used in patient trials assessing safety and efficacy for evidence for FDA approval.
Recurrent prostate cancer
Standard Therapies for Treatment of Recurrent Prostate Cancer
Traditional curative therapy for prostate cancer has been either surgical resection or radiotherapy. Patients are roughly divided with half choosing surgery and half choosing radiotherapy. Recurrences after surgical resection can range from 25% to 40%, which is usually manifested by increasing serum level of PSA. Approximately 30,000 men develop biochemical recurrence (BCR) with increasing PSA levels after radical prostatectomy each year in the United States. One study examining where recurrences occur found that 81% had a local prostate bed recurrence that could be shown with MR imaging using an endorectal coil. For those undergoing radiotherapy, BCR can range widely, from 33% to 63%, over 10 years, and contributes another 45,000 men per year with recurrent cancer from radiotherapy in the United States. Although 5-year disease-free survival from prostate cancer, including good outcomes from primary therapies, approaches 100% in the United States, these figures clearly show that many men develop recurrent cancer each year. Salvage treatments for recurrent prostate cancer include salvage radical prostatectomy (sRP), salvage radiotherapy, salvage US-guided HIFU, salvage US-guided cryoablation, and newly described salvage MR imaging–guided laser and cryoablation.
Limitations of Current Salvage Therapies for Recurrent Prostate Cancer
Surgery
sRP after radiotherapy is more difficult because of local fibrosis and tissue plane obliteration secondary to the radiation, with limited centers of excellence offering this surgery. Because of the difficulties posed after primary radiation treatment failure, the complication rates for sRP have been higher than primary surgery, with incontinence rates of 58% and major complication rates of 33%.
Radiation
Salvage radiotherapy can be used for BCR after surgery or primary radiotherapy failures. In a large study at the Mayo Clinic, 49 patients with primary external beam radiotherapy failure were treated with salvage low-dose rate brachytherapy. They showed a 3-year biochemical disease-free survival (bDFS) of 48% and a 5-year bDFS of 34%. Complications for salvage brachytherapy were either genitourinary or gastrointestinal. Grade 3 to 4 genitourinary toxicity was 17% as a late complication, and grade 3 to 4 gastrointestinal toxicity was approximately 5.6%.
High-intensity focused ultrasonography
Salvage US-guided HIFU has been used for salvage therapy. Three different studies have been published, with relatively short follow-up periods of 7.4 to 18.1 months. These studies reported a highly variable bDFS of 25% to 71%, which was confounded by variable definitions of PSA failure and variable use of hormonal therapy before treatment. The most commonly reported complications are incontinence (10%–49.5%), urethral stricture with retention (17%–17.6%), erectile dysfunction (66.2%–100%), and rectourethral fistula (3%–16%).
Salvage ultrasound-guided cryotherapy
US-guided cryotherapy was used for salvage therapy in several limited studies. The most recent large study from the Cryo On-Line Data (COLD) registry reported a 5-year bDFS of 58.9% by the American Society for Radiation Oncology definition of BCR and 54.5% by the Phoenix definition of BCR. For patients treated with salvage US-guided cryotherapy after primary radiotherapy failure, the most recent reported complication rates are perineal pain (4%–14%), mild-moderate incontinence (6%–13%), severe incontinence (2%–4%), and urethrorectal fistula (1%–2%). With the use of a urethral warming catheter, the rate of sloughing and urethral stricture has been reduced to near zero. Erectile dysfunction is still high, with rates of 69% to 86%.
Selection of Patients with Recurrent Prostate Cancer for Magnetic Resonance–Guided Focal Therapy
The first issue is to determine whether the increasing PSA levels represent local recurrence, systemic recurrence, or both. The second issue in managing patients with BCR of prostate cancer is assessing the risk of cancer treatment versus the risk of further intervention. Overall, rapid increase in PSA levels, short disease-free interval, and high-grade disease are all poor prognostic indicators, with a higher probability of systemic recurrence, whereas slow increase in PSA levels, long disease-free interval, and low-grade disease are better prognostic indicators, with a higher probability of local recurrence.
Suggested criteria for MR-guided focal ablative treatment in recurrent prostate cancer are (1) biopsy-proven local recurrent tumor that can be visualized by MR imaging, (2) absence of distant metastasis confirmed with chest, abdomen, pelvis CT, or MR imaging plus bone scintigraphy, or [ 11 C]choline PET/CT scan. Although these selection criteria are not perfect, they are helpful in avoiding treatment of what is believed to be a local recurrence that is really systemic.
Magnetic resonance–guided cryoablation
MR-guided cryoablation for recurrent prostate cancer has also been shown feasible and successful in several small limited studies. Woodrum and colleagues published a study of 18 patients treated with MR-guided cryoablation for locally recurrent prostate cancer. These investigators broke the cohort into 2 groups of 9 patients each, with alternation of the cryoablation technique between the groups. They reported that tight (5 mm) spacing, 3 freeze–thaw cycles, and sometimes decreasing the urethral warmer temperature produced better short-term recurrence-free intervals. In addition, Gangi and colleagues reported successful MR-guided cryoablation treatment of several patients with recurrent prostate cancer. Using MR guidance, cryoablation treatment can be tailored to the desired region ( Fig. 2 ) or focal area ( Fig. 3 ).