Contrast-enhanced breast MR imaging is increasingly being used to diagnose breast cancer and to perform biopsy procedures. The American Cancer Society has advised women at high risk for breast cancer to have breast MR imaging screening as an adjunct to screening mammography. This article places special emphasis on biopsy and operative planning involving MR imaging and reviews use of breast MR imaging in monitoring response to neoadjuvant chemotherapy. Described are peer-reviewed data on currently accepted MR imaging–guided procedures for addressing benign and malignant breast diseases, including intraoperative imaging.
Key points
- •
Breast MR imaging is the most sensitive imaging tool of detecting breast cancer and may reveal breast cancer that is occult to physical examination and by conventional imaging modalities (mammography and ultrasound).
- •
In cases in which a suspicious lesion is detected by MR imaging and no obvious correlative finding is found by other methods, MR imaging–guided tissue sampling is needed to determine the underlying histopathology.
- •
Studies have shown advantages of breast MR imaging for predicting recurrence-free survival and pathologic complete response over physical examination and conventional imaging.
- •
Regarding lumpectomy planning, anticipated benefits from higher sensitivity of preoperative MR imaging have not been clearly shown in large studies.
Problem and clinical presentations
Use of Contrast-Enhanced Breast MR Imaging
Contrast-enhanced breast MR imaging is an important adjunctive modality for screening and diagnosis of breast cancer. MR imaging has been demonstrated as beneficial and used increasingly as an adjunct to mammography in screening in a subset of women at high risk for developing breast cancer because of its high sensitivity and negative predictive value. MR imaging is being used to assess response for neoadjuvant chemotherapy treatment (NACT), detect otherwise occult breast cancer presenting as metastatic axillary or systemic disease, evaluate extent of disease in patients with newly diagnosed breast cancer, and assess contralateral breast. Additional clinical trials are needed to determine the significance of MR imaging–detected, otherwise occult disease.
MR Imaging–Guided Tissue Sampling
In cases in which MR imaging alone detects a suspicious lesion (ie, no correlative finding with other methods), MR imaging–guided tissue sampling is needed to determine the underlying histopathology.
Margin Status at Breast-Conserving Therapy
The current positive or close margin rate at initial surgery requiring an additional operation with re-excision is estimated to range from 30% to 60%. There is no ideal method for margin evaluation during surgery. However, there are trials in progress on the use of MR imaging guidance and MR imaging evaluation of the margins intraoperatively with the goal of reducing the need for additional operations.
Problem and clinical presentations
Use of Contrast-Enhanced Breast MR Imaging
Contrast-enhanced breast MR imaging is an important adjunctive modality for screening and diagnosis of breast cancer. MR imaging has been demonstrated as beneficial and used increasingly as an adjunct to mammography in screening in a subset of women at high risk for developing breast cancer because of its high sensitivity and negative predictive value. MR imaging is being used to assess response for neoadjuvant chemotherapy treatment (NACT), detect otherwise occult breast cancer presenting as metastatic axillary or systemic disease, evaluate extent of disease in patients with newly diagnosed breast cancer, and assess contralateral breast. Additional clinical trials are needed to determine the significance of MR imaging–detected, otherwise occult disease.
MR Imaging–Guided Tissue Sampling
In cases in which MR imaging alone detects a suspicious lesion (ie, no correlative finding with other methods), MR imaging–guided tissue sampling is needed to determine the underlying histopathology.
Margin Status at Breast-Conserving Therapy
The current positive or close margin rate at initial surgery requiring an additional operation with re-excision is estimated to range from 30% to 60%. There is no ideal method for margin evaluation during surgery. However, there are trials in progress on the use of MR imaging guidance and MR imaging evaluation of the margins intraoperatively with the goal of reducing the need for additional operations.
Need for MR imaging–guided procedures
Recommendations for performance of breast MR imaging are conditioned on a standard level of quality of MR imaging studies with high spatial resolution images. The American College of Radiology accreditation process includes the requirement for facilities to have the ability to provide MR imaging–guided biopsy when offering breast MR imaging.
When a suspicious lesion has been detected by breast MR imaging, and biopsy for histologic diagnosis is suggested, the first step should be to evaluate the area by mammography and targeted ultrasound (US) for a possible correlate. US guidance is preferred over MR imaging for biopsy if a sonographic correlate can be identified. US is readily available and US-guided biopsies are quicker, more comfortable for the patient, do not require intravenous contrast, and are less expensive. A US correlate can be identified in approximately half of the cases. If the findings of this approach are unrevealing or uncertain, an MR imaging–guided biopsy should be performed.
Breast MR imaging and techniques
There are widespread variations in breast MR imaging techniques, with different approaches to balance morphology, kinetic information, and use of fat saturation versus subtraction techniques. Obtaining good quality breast MR imaging is conditioned on many factors: use of a high-field-strength magnet and a dedicated breast coil, appropriate breast positioning, injection of gadolinium contrast material, high-spatial-resolution imaging without artifacts, and specified adequate timing of the dynamic sequences.
The following MR imaging equipment specifications and performance must meet all state and federal requirements, and the American College of Radiology practice parameters and technical standards guidelines including routine quality control should apply. Field strength: a 1.5- or 3-T magnet has typically been used for breast MR imaging. Positioning: all routine clinical breast MR imaging examinations are performed with the patient in prone position with simultaneous bilateral imaging using a dedicated (bilateral) breast MR imaging coil containing two individual depressions for the left and right breast. Prone positioning helps to move the breasts away from the chest wall and minimizes respiratory and cardiac motion effects. Resolution, contrast, and artifacts: the slice thickness should be 3 mm or less; in-plane pixel resolution should be 1 mm or less so as to reduce the problem of volume averaging and to detect and characterize small abnormalities. Chemical fat suppression is helpful as a method for reducing the fat signal. Subtraction imaging for assessment of enhancement and fat suppression are recommended. Misregistration caused by patient motion can occur, and motion correction may aid in reducing artifacts encountered with image subtraction. Contrast: gadolinium intravenous contrast is needed in the evaluation of breast cancer. Dynamic kinetic information based on enhancement data at appropriate time intervals is extremely important for lesion classification.
Challenges in MR imaging–guided breast biopsy targeting
Many of the challenges experienced with MR imaging–guided biopsy are similar to those encountered using stereotactic biopsy with patients prone on a dedicated table and are related to targeting (ie, difficulty with posterior targets or those that are superficial), positioning, and compression (eg, an accordion effect at clip deployment or problems with very dense breasts). Furthermore with MR imaging-guidance, the patient needs to be removed from the magnet to be repositioned for the biopsy to be performed, because there is somewhat limited access to the medial and posterior breast. Additional difficulties may arise, including contrast washout, lesion location-related problems, and/or limitations in confirming lesion sampling.
Cancellation of the procedure is frequent (reported as between 8% and 13%). Nonvisualization of the suspicious finding may be caused by change in tissue enhancement because the patient is in different phase of her period and/or may be related to compression of breast tissue with decreased inflow of contrast material.
Signal-void artifact from needles, obturators, and wires used in the MR imaging setting and hemorrhage (hyperintense on T1 sequences) may obscure the target. Air entered or generated from needle placement frequently interferes with target visualization.
MR imaging–guided breast core needle biopsy
A dedicated breast MR imaging coil and prone positioning on a moveable examination table is typically required with MR imaging conditional biopsy equipment. Usually a larger needle (11–14 gauge) and vacuum assistance are used for sampling, although smaller, spring-activated 14- to 18-gauge sizes are also available. Needle susceptibility artifact should be reduced by appropriate imaging protocol without compromising image quality and lesion detection.
The grid technique is widely implemented because of its ease of use. Other localizing methods include pillar and post, and free-hand techniques. Protocols may differ among facilities. Usually, after localizing images, axial and sagittal T1-weighted, fat saturated images are obtained before and after injection of the contrast agent in the area of interest. The imaging protocol should minimize image acquisition time while maintaining lesion visualization. There is a short period following the administration of the intravenous contrast during which the area of interest can be visualized. Following identification, targeting the lesion includes identifying the correct opening within the grid for introducer and for needle insertion. Most systems offer an approach from the lateral, or from the medial direction. There is frequently tissue displacement during the needle insertion and repeated adjustments in needle positioning may be required ( Fig. 1 ). Placing a marker clip at the biopsy site, typically followed by two mammographic views to document clip location, is recommended. Vacuum-assisted core biopsy tissue sampling with MR imaging–guided devices has been shown to be technically successful in 94% to 98% of cases and is an accepted alternative for histopathologic assessment to surgical biopsy.
MR imaging–guided wire localization
The first MR imaging–guided interventional procedure developed was needle localization before surgery. The procedure is occasionally performed when the extent of disease is not apparent by conventional imaging modalities, and therefore prelumpectomy localization is best done with MR imaging. Currently, MR imaging–guided core biopsy has replaced many MR imaging needle localizations. Excision is sometimes considered when core biopsy is not possible (eg, there is a posterior target location or an extremely small breast) or per patient’s preference.
The positioning and targeting for needle localization is the same as that for needle biopsy. After lesion identification and location determination, a guide needle is introduced to the appropriate depth. After imaging confirms appropriate location and depth, an MR imaging conditional localization hook wire is deployed through the needle. The guiding needle is similar to the Kopans needle used for mammographic localizations. The MR imaging wire is softer than conventional, non–MR imaging conditional wires and therefore deployment in hard fibrous tissue may occasionally be difficult and they have a tendency to break during surgery. Following the localization procedure, a mammogram can visualize for the surgeon the site of the wire within breast tissue, and nipple and chest-wall positions.
Pathology correlation of MR imaging–guided biopsies
Evaluating concordance is important in all image-guided biopsies, and especially important for MR imaging–guided biopsies because sampling accuracy is subject to uncertainty. Concordance decisions begin in the planning phase with the radiologist defining the expected pathology result based on original images. Because there is no specimen image confirmation of the target (as in a stereotactic core biopsy specimen radiograph showing calcifications), or direct visualization of sampling (as in US-guided biopsies with real-time observation of sampling), accuracy is difficult to determine from core biopsy images. The procedure radiologist should review images to determine whether procedure images support lesion retrieval. The final decision about concordance versus discordance is made when the radiologist decides if pathology results agree with the expected outcome.
Based on the radiologist’s degree of certainty regarding satisfactory tissue sampling, benign concordant histologic results may warrant short-term (6-month) follow-up MR imaging to confirm stability. For discordant lesions, surgical excision is recommended. Imaging histologic discordance rate has been reported as approximately 7% to 9%. Higher rates of imaging-histologic discordance and underestimation of atypical ductal hyperplasia and ductal carcinoma in situ (DCIS) have been reported with MR imaging–guided biopsies than with stereotactic mammographic biopsies.
For cases that have been assessed as possibly missed or discordant, repeat biopsy or surgical excision is recommended. MR imaging–guided core biopsy malignancy rates varying between 16% and 37% have been reported.
Regarding pathology examination of excisional biopsy specimen of MR imaging–guided wire localization, MR imaging of a breast specimen with current clinical scanners is not useful for lesion detection because detection is based on visualization of enhancement with the injected contrast agent. Gross examination and specimen radiography do not identify most of the malignancies in MR imaging localized procedures. For that reason, optimal pathology processing of MR imaging–guided excisions requires microscopic examination of the entire specimen tissue.
Surgical planning with preoperative MR imaging following neoadjuvant chemotherapy
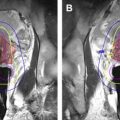
Systemic chemotherapy improves survival for patients with invasive breast cancer. It is the standard of care for node-positive patients and is used for many patients with high-risk node-negative disease with invasive breast cancer. During the past approximately 20 years, there has been an option to administer chemotherapy before surgery (NACT) rather than following surgery (adjuvant chemotherapy) for those women requiring systemic therapy. The main advantage of preoperative NACT is the reduction in primary tumor size and conversion from node-positive into node-negative status. NACT is used for treatment of locally advanced breast cancer to allow for surgery in cases in which skin or pectoral muscle is involved ( Fig. 2 ). NACT is also used in early stage breast cancer to enable breast-conserving therapy (BCT) when originally mastectomy was planned, or to achieve better cosmetic outcomes because of smaller surgical resection volume. Despite less extensive surgery following NACT, several studies showed similar local recurrence rates with preoperative NACT compared with adjuvant chemotherapy, although some studies suggested a trend for higher locoregional recurrence. The National Surgical Adjuvant Breast and Bowel Project B18 and other clinical trials comparing neoadjuvant with adjuvant chemotherapy found that there is no significant difference in overall or disease-free survival between patients receiving adjuvant or neoadjuvant chemotherapy; however, more women undergoing preoperative chemotherapy were eligible and received breast-conservation treatment.
Accurate monitoring of NACT response is essential; imaging may demonstrate stable or progressive disease, or remission, and even complete response ( Box 1 ). Pathologic complete response is defined as the absence of any residual invasive tumor cells in the original tumor bed; however, residual DCIS may be present ( Fig. 3 ). Attaining pathologic complete response following NACT has been shown as a prognostic factor for overall better survival, and for disease-free survival.
- •
The amount of residual invasive cancer following therapy is an important prognostic predictor.
- •
Pathologic complete response is consistently associated with a favorable outcome, especially in estrogen receptor negative (ERBB2 [Her-2/neu] positive and triple negative) tumors.
- •
Close monitoring of tumor response is required.
- •
Best monitoring modality: dynamic contrast-enhanced breast MR imaging.
- •
Data have shown that MR imaging is superior to clinical examination and other breast imaging methods regarding accuracy and positive predictive value in determining postneoadjuvant chemotherapy pathologic tumor response.
- •
Accuracy of MR imaging is highest in estrogen receptor/progesterone receptor (PR) negative (ERBB2 [HER2/NEU] positive and triple negative) tumors ( Fig. 4 ).
MR imaging–guided breast ablation
The aim of ablative therapy is to achieve a well-defined area encompassing the tumor, irreversible cell damage, protein denaturation, and coagulation necrosis, while sparing overlying and surrounding tissues. The role of imaging is to aid the clinician in planning the probe placement for optimal coverage, targeting the lesion, and monitoring the deposition of energy. The advantages of MR imaging guidance in these tasks are three-dimensional visualization via multiplanar, multislice acquisition, high sensitivity, and delineation of breast lesions, and tissue thermal sensitivity. A therapeutic probe is percutaneously placed in the lesion to deliver cooling (cryoablation) or heating energy (radiofrequency ablation [RFA], laser interstitial thermal therapy [LITT]) so as to cause cell death. High-intensity focused US (HIFU) can achieve these goals without use of an invasive probe. Ablative techniques may be useful in patients with benign lesions, those who refuse surgery, patients with stage 4 breast cancer who need palliative care, or patients with recurrent disease.
There are uncertainties that may prevent image-guided minimally invasive tumor ablation in patients with early stage breast cancer from becoming a viable alternative treatment of lumpectomy. It remains to conclusively show that clinical outcomes (clear margins, recurrence rate, morbidity, and mortality) are comparable with the standard of care, surgery followed by whole breast radiation. Careful inclusion criteria and control measures are critical elements.
MR Imaging–Guided Cryoablation
Percutaneous cryoablation using freezing temperatures is delivered by gas-cooled probes. Although most breast ablation has been guided by US, MR imaging is particularly well suited for monitoring the growth of the iceball. The iceball appears as a signal void because of the short T2* of the crystalized water and, unlike with US, the tissue beyond the iceball is not subject to shadowing.
In a feasibility study, Morin and colleagues reported on the MR imaging–guided cryoablation in 25 patients with breast carcinoma without complications. Four weeks after treatment, surgical excision was performed for histopathologic correlation. Total ablation was achieved in 13 of the 25 tumors treated. Pusztaszeri and colleagues reported that in all 10 of the evaluated patients undergoing MR imaging–guided cryotherapy followed by surgical excision, the iceball engulfed the tumor, but only two patients had a complete response. The authors suggested that components of undetected DCIS in the larger tumors were far from the two probes used. Five patients suffered from skin necrosis, a complication that can be avoided by selection criteria of minimum distance between the lesion and the skin or managed with the use of warm saline on the skin or saline injection. In these studies, the patient was supine. More recently, Tozaki and colleagues treated a single patient with core needle biopsy proved invasive ductal carcinoma without an intraductal component using a non–MR imaging compatible cryotherapy system. MR imaging of a prone patient with a breast coil was used to define the target tissue. A US system safely integrated into the MR imaging room was used to place the probes. At the 9-week MR imaging evaluation, the lesion was not enhancing and was shown to be inside the cryozone. No viable cancer cells were noted on histology following a lumpectomy at 14 weeks.
MR Imaging Temperature Mapping in the Breast
A tool common to the ablative techniques that use elevated temperature is noninvasive MR imaging temperature mapping based on temperature-sensitive MR imaging parameters, such as the proton resonance frequency, the diffusion coefficient, T1 and T2 relaxation times, magnetization transfer, proton density, and temperature-sensitive contrast agents. Through empirical experimentation, cell death can be correlated with thermal dose, which is derived from time-temperature curves. Although proton resonance frequency shift is useful for measuring temperature in aqueous tissue, the chemical shift in fat is almost constant with the temperatures used in thermal ablation. However, the T1 temperature dependence can be exploited in fat.
MR Imaging–Guided Radiofrequency Ablation
RFA refers to the destruction of tissue via the application of electromagnetic fields created by interstitial electrode delivery of energy (0.4–8 MHz). A dispersive electrode on the thigh or back is used to complete the electrical circuit. Current density is induced in the tissue, causing resistive heating. RF energy deposition is a function of tissue conductivity and is difficult to predict and control. The formation of the thermal lesion may be inhomogeneous, especially in regions of the tissue boundaries. Susceptibility artifacts around the probe during MR imaging may prevent accurate temperature monitoring. No monopolar commercial solution is currently available to remedy the problem of electromagnetic interference emitting from the RF generator manifesting as noise in the MR images. Several research sites have implemented gating or filtering solutions.
van den Bosch and colleagues performed MR imaging–guided RFA on three patients followed immediately by surgical excision for histopathologic correlation ( Fig. 5 ). US-guided large-core needle biopsy confirmed invasive ductal carcinoma in all three patients, with DCIS adjacent to the invasive lesion in the second and third patients. Patients were positioned prone in a 0.5-T vertically open MR imaging scanner. Measurements from a fiberoptic temperature probe were used for comparisons with MR imaging temperature mapping. Histopathology confirmed successful (100%) tumor ablation in one patient, and partial tumor destruction (33% and 50%, respectively) in two patients. The lesion size was probably underestimated on the MR image in the latter two cases. It was noted that susceptibility artifact caused by the 6-mm diameter probe would create a challenge for temperature mapping in lesions less than 10 mm. A high success rate for the technique in other organs may encourage industry to provide complete solutions for breast MR imaging–guided RFA.