MR imaging–guided interventions for treatment of low back pain and for diagnosis and treatment of soft tissue and bony spinal lesions have been shown to be feasible, effective, and safe. Advantages of this technique include the absence of ionizing radiation, the high tissue contrast, and multiplanar imaging options. Recent advancements in MR imaging systems allow improved image qualities and real-time guidance. One exciting application is MR imaging–guided cryotherapy of spinal lesions, including treating such lesions as benign osteoid osteomas and malignant metastatic disease in patients who are not good surgical candidates. This particular technique shows promise for local tumor control and pain relief in appropriate patients.
Key points
- •
The availability of MR imaging–guided spine interventions for back pain is currently limited, but interest and expertise are growing.
- •
MR imaging–guided interventions for back pain can be beneficial because of superior visualization of soft tissues and fluid, and absence of exposure to ionizing radiation.
- •
MR imaging–guided interventions for diagnosis and treatment of soft tissue and bony spinal lesions are safe and effective because of their superior visualization of soft tissues and fluid enabling avoidance of injury to adjacent critical structures and absence of exposure to ionizing radiation.
- •
The availability of MR imaging–guided spine interventions for diagnosis and treatment of spinal lesions currently continues to be limited because of the equipment costs and lack of universal expertise.
- •
These costs will probably play an increasing role in the future for treatment of solid organ masses.
Definition of problem and clinical presentation
The information available regarding procedural strategies for MR imaging–guided spine interventions is currently limited compared with that of more conventional radiation-based image-guidance techniques, such as fluoroscopy; however, interest and expertise are growing. With ongoing advancements in MR imaging techniques, new applications and opportunities are emerging, including those for treatment of low back disorders.
Low back disorders are widely prevalent. Low back pain is the leading cause of occupational disability worldwide, with lifetime adult prevalence varying from 50% to 80%. Percutaneous image-guided spinal injections with corticosteroids and anesthetics are established therapeutic methods for low back pain. These procedures are traditionally performed with proved safety and accuracy under fluoroscopy or computed tomography (CT). Real-time CT guidance affords improved targeting because it allows cross-sectional visualization of critical anatomy, facilitates freehand real-time needle placement, and has the potential for more accurate placement into facet joints. However, there is an unavoidable risk of ionizing radiation associated with these techniques. This is a concern, particularly when these procedures are performed on young individuals of fertile age and serial therapeutic procedures are required.
MR imaging is an emerging modality in the guidance of various minimally invasive therapeutic interventions for low back pain because of the absence of ionizing radiation, the high soft tissue and fluid contrast, and multiplanar imaging options it provides. Newly developed MR imaging systems have been used in a variety of spine procedures for back pain including nerve root injection, facet joint injection, epidural injection, and facet joint neurotomy using cryotherapy. MR imaging–guided spine interventions are also available for the treatment and diagnosis of pathologic conditions, including biopsy and treatment of primary spinal tumors and metastatic disease.
Because MR imaging is superior to other imaging techniques in depicting soft tissue and bone lesions in the spine, it is sometimes advantageous to use MR imaging when performing percutaneous biopsies. It allows for the accurate targeting of the lesion to be biopsied and clearly depicts critical structures that need to be carefully navigated to reach the target. In addition, MR imaging often provides superior depiction of the internal composition of lesions, allowing for targeted site-specific biopsies of heterogeneous lesions. Therefore, MR imaging can be used full circle with the detection of the initial lesion, planning and guidance for the biopsy of the lesion, and monitoring of treatment response following biopsy. MR imaging–guided biopsy does, however, require specialized biopsy equipment that is compatible with the MR imaging environment and that has limited susceptibility artifact.
New applications of MR imaging–guided spine interventions are emerging, especially in the treatment of patients that are considered poor surgical candidates. Approximately one-half of the patients with metastatic disease have poorly controlled pain. Frequently, these patients have exhausted conventional therapies; therefore, percutaneous treatments have emerged to reduce pain, allow for local tumor control, and improve quality of life. MR imaging–guided interventions reduce morbidity compared with that of surgery and other conventional techniques, such as radiation therapy, by using a percutaneous approach with only one to a few tiny skin punctures needed to insert the biopsy needles or treatment probes.
One emerging treatment option is MR imaging–guided cryotherapy for the treatment of bone and soft tissue metastases and some primary tumors. Because the MR imaging signal is temperature sensitive, MR imaging can be used to monitor thermal ablation that during cryotherapy is visualized as an area of decreased signal intensity surrounded by a rim of hyperintensity on T2-weighted images, often referred to as an “iceball.” The shape and size of the cryotherapy iceball is easily monitored and can be tailored in real time using multiple planes on MR imaging. MR imaging, well known as far superior to other imaging techniques for the depiction of soft tissue structures, can enable superior visualization of the critical structures, often near lesions in the spine, including spinal nerve roots, vasculature, and spinal cord with surrounding cerebral spinal fluid (CSF), which can be well-visualized in comparison with the iceball treatment zone. Tumors typically have increased signal intensity on T2-weighted images, whereas iceballs cause a signal void; therefore, the coverage of the treatment zone in relation to the extent of tumor is normally easily depicted. MR imaging is ideal for tailoring the iceball treatment zone to cover as much of the lesion as possible without damaging nearby critical soft tissues. Because of these advantages, MR imaging–guided percutaneous cryotherapy for both soft tissue and bone spinal metastatic disease and primary tumors has been shown to be safe and feasible in anatomic locations adjacent to critical structures.
Although there are inherent advantages of MR imaging as a guidance modality, there are also several disadvantages. There are fewer choices of MR imaging–compatible equipment and these often cost more than conventional equipment. MR imaging–guided procedures have the potential for being more time consuming and, therefore, more costly overall. However, faster imaging sequences with single images obtained on the order of 1 second and the advancement of actual real-time monitoring systems of the needle position are helping to significantly reduce the time required for MR imaging–guided biopsies. In addition, there is a myriad of patient-specific contraindications to the MR imaging environment, including the presence of pacemakers or other non–MR imaging compatible medical devices or foreign bodies.
Definition of problem and clinical presentation
The information available regarding procedural strategies for MR imaging–guided spine interventions is currently limited compared with that of more conventional radiation-based image-guidance techniques, such as fluoroscopy; however, interest and expertise are growing. With ongoing advancements in MR imaging techniques, new applications and opportunities are emerging, including those for treatment of low back disorders.
Low back disorders are widely prevalent. Low back pain is the leading cause of occupational disability worldwide, with lifetime adult prevalence varying from 50% to 80%. Percutaneous image-guided spinal injections with corticosteroids and anesthetics are established therapeutic methods for low back pain. These procedures are traditionally performed with proved safety and accuracy under fluoroscopy or computed tomography (CT). Real-time CT guidance affords improved targeting because it allows cross-sectional visualization of critical anatomy, facilitates freehand real-time needle placement, and has the potential for more accurate placement into facet joints. However, there is an unavoidable risk of ionizing radiation associated with these techniques. This is a concern, particularly when these procedures are performed on young individuals of fertile age and serial therapeutic procedures are required.
MR imaging is an emerging modality in the guidance of various minimally invasive therapeutic interventions for low back pain because of the absence of ionizing radiation, the high soft tissue and fluid contrast, and multiplanar imaging options it provides. Newly developed MR imaging systems have been used in a variety of spine procedures for back pain including nerve root injection, facet joint injection, epidural injection, and facet joint neurotomy using cryotherapy. MR imaging–guided spine interventions are also available for the treatment and diagnosis of pathologic conditions, including biopsy and treatment of primary spinal tumors and metastatic disease.
Because MR imaging is superior to other imaging techniques in depicting soft tissue and bone lesions in the spine, it is sometimes advantageous to use MR imaging when performing percutaneous biopsies. It allows for the accurate targeting of the lesion to be biopsied and clearly depicts critical structures that need to be carefully navigated to reach the target. In addition, MR imaging often provides superior depiction of the internal composition of lesions, allowing for targeted site-specific biopsies of heterogeneous lesions. Therefore, MR imaging can be used full circle with the detection of the initial lesion, planning and guidance for the biopsy of the lesion, and monitoring of treatment response following biopsy. MR imaging–guided biopsy does, however, require specialized biopsy equipment that is compatible with the MR imaging environment and that has limited susceptibility artifact.
New applications of MR imaging–guided spine interventions are emerging, especially in the treatment of patients that are considered poor surgical candidates. Approximately one-half of the patients with metastatic disease have poorly controlled pain. Frequently, these patients have exhausted conventional therapies; therefore, percutaneous treatments have emerged to reduce pain, allow for local tumor control, and improve quality of life. MR imaging–guided interventions reduce morbidity compared with that of surgery and other conventional techniques, such as radiation therapy, by using a percutaneous approach with only one to a few tiny skin punctures needed to insert the biopsy needles or treatment probes.
One emerging treatment option is MR imaging–guided cryotherapy for the treatment of bone and soft tissue metastases and some primary tumors. Because the MR imaging signal is temperature sensitive, MR imaging can be used to monitor thermal ablation that during cryotherapy is visualized as an area of decreased signal intensity surrounded by a rim of hyperintensity on T2-weighted images, often referred to as an “iceball.” The shape and size of the cryotherapy iceball is easily monitored and can be tailored in real time using multiple planes on MR imaging. MR imaging, well known as far superior to other imaging techniques for the depiction of soft tissue structures, can enable superior visualization of the critical structures, often near lesions in the spine, including spinal nerve roots, vasculature, and spinal cord with surrounding cerebral spinal fluid (CSF), which can be well-visualized in comparison with the iceball treatment zone. Tumors typically have increased signal intensity on T2-weighted images, whereas iceballs cause a signal void; therefore, the coverage of the treatment zone in relation to the extent of tumor is normally easily depicted. MR imaging is ideal for tailoring the iceball treatment zone to cover as much of the lesion as possible without damaging nearby critical soft tissues. Because of these advantages, MR imaging–guided percutaneous cryotherapy for both soft tissue and bone spinal metastatic disease and primary tumors has been shown to be safe and feasible in anatomic locations adjacent to critical structures.
Although there are inherent advantages of MR imaging as a guidance modality, there are also several disadvantages. There are fewer choices of MR imaging–compatible equipment and these often cost more than conventional equipment. MR imaging–guided procedures have the potential for being more time consuming and, therefore, more costly overall. However, faster imaging sequences with single images obtained on the order of 1 second and the advancement of actual real-time monitoring systems of the needle position are helping to significantly reduce the time required for MR imaging–guided biopsies. In addition, there is a myriad of patient-specific contraindications to the MR imaging environment, including the presence of pacemakers or other non–MR imaging compatible medical devices or foreign bodies.
Anatomy
MR imaging is often much better at depicting lesions and surrounding normal anatomic structures because of its superior contrast resolution and often superior spatial resolution compared with CT. Vessels are typically easily identified on most MR pulse sequences as are other critical structures, including nerve roots, thecal sac, CSF, and the spinal cord. Especially with biopsies of the spine, needle tip positions in the extradural, intradural/extramedullary, and intramedullary spaces can be readily identified on MR imaging sequences. Multiplanar two-dimensional MR imaging sequences and three-dimensional MR imaging sequences with multiplanar reformats provide high spatial resolution depiction of the anatomy in any desired imaging plane.
Similar to fluoroscopy- and CT-guided spine injections, the targets of MR imaging–guided spine injections include nerve roots, facet joints, and epidural space. Selective nerve root injections are performed with percutaneous transforaminal drug delivery into the spinal nerve sheath. Facet joint injection is performed with percutaneous injection of the drug into the lumbar facet joint space. Epidural injection is performed with percutaneous translaminar injection of the drug into the epidural space through a posterior intervertebral approach. Nontarget injection, including intravascular injection and injection into the subarachonoid space, is avoided with either real-time monitoring using fast MR imaging sequences or with aspiration before drug delivery.
Imaging protocols and imaging findings
There has been a variety of MR imaging systems and configurations used for MR imaging–guided spinal interventions with greater emphasis now on the use of higher 3.0-T field strength systems. Higher-resolution spinal imaging and sequences with increased imaging speed are widely available on 3.0-T magnets. Wider-bore magnets (or typically lower field strength open-bore systems) allow for manipulation of the interventional equipment without moving the patient. After the initial high-resolution standard MR imaging sequences are obtained for lesion identification and planning, fast acquisition T1- or T2-weighted sequences are used for quick monitoring of needle advancement to the lesion of interest. If needed, specialized sequences that oversample the center of k-space (BLADE or PROPELLOR sequences) can be used to reduce patient motion artifact. In patients with spinal hardware, advanced MR imaging techniques, such as slice encoding for metal artifact correct and multiacquisition variable-resonance image combination, can be used to minimize metallic artifacts.
MR imaging–guided spine interventions for low back pain have been performed in a few different MR imaging systems. In the past, MR imaging–guided spine interventions were performed in dedicated open low-field-strength MR imaging systems. The low-field systems, however, had limited capability in providing real-time fluoroscopic guidance. More recently, a wide-bore high-field MR imaging system and an open high-field system with vertical field orientation have been used in spine interventions for low back pain. These novel high-field systems yield real-time or near real-time guidance and improved contrast-to-noise ratio.
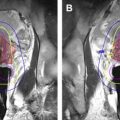
Fritz and colleagues described the use of a 1.5-T open MR imaging system (Magnetom Espree, Siemens, Medical Solutions, Malvern, PA) with patients in prone position for different procedures including nerve root injection and facet joint injection ( Figs. 1 and 2 ). For the diagnostic imaging phase of the procedure, this group used a body matrix coil with parallel imaging technology. The coil was then exchanged for a flexible loop coil for the interventional phase. Each procedure started with axial T1-weighted turbo spin echo imaging for planning of a needle path for direct access to target structures. For MR fluoroscopic determination of skin entry site, continuously acquired and displayed single-slice T1/T2*-weighted fast low angle shot (FLASH) two-dimensional MR imaging or T2-weighted fast imaging with steady-state precession was used as a syringe filled with saline solution or gadolinium-enhanced saline solution was moved over the skin. Following antiseptic preparation of the skin, draping, and induction of local anesthesia, the T1/T2*-weighted FLASH two-dimensional MR imaging sequence was used to navigate the puncture needle to the target structure with real-time MR imaging guidance. Injectants used in this study contained gadolinium-based contrast material. Following drug injection, T1-weighted imaging with fat suppression was performed for visualization of fluid distribution.