Semiquantitative assessment of the knee by expert magnetic resonance imaging readers is a powerful research tool for understanding the natural history of osteoarthritis (OA). Several reliable semiquantitative scoring systems have been applied to large observational cross-sectional and longitudinal epidemiologic studies and interventional clinical trials. Such evaluations have enabled understanding of the relevance of disease in structures within the knee joint to explain pain and progression of OA. Compositional imaging of cartilage has added to our ability to detect early degeneration before morphologic changes are present, which may help to prevent the permanent morphologic changes commonly seen in knee OA.
Over the past 2 decades magnetic resonance (MR) imaging has become established as the most important imaging modality in the assessment of joint disease in both the clinical and research environments. Regarding knee osteoarthritis (OA), MR imaging-based studies first focused on the assessment of hyaline articular cartilage as the main outcome measure in clinical and epidemiologic studies. However, it is widely accepted now that OA is a disease of the whole joint including the subchondral bone, synovium, menisci, and ligaments. Because MR imaging is able to depict all tissues of the joint directly, the joint can be assessed as a whole organ, providing a detailed picture of the structural changes associated with OA, as well as the role of such changes in predicting pain and progression of disease. Validated MR imaging-based semiquantitative (SQ) scoring systems are available for the assessment of the whole knee joint in OA and have been applied to many OA studies. The analyses based on SQ scoring have added deeply to our understanding of the physiopathology and natural history of knee OA, as well as to the clinical implications of the structural changes.
Several MR imaging techniques are available to evaluate composition of hyaline cartilage, and degenerative changes can be detected before morphologic changes are seen. These techniques have increased our understanding of early and potentially reversible disease, and may help to avoid the permanent morphologic changes commonly seen in knee OA. This review discusses MR imaging-based SQ scoring systems for the evaluation of knee OA, focusing on the role of different intra-articular structural pathologies in predicting pain and progression of disease. The review also discusses available MR imaging techniques for the assessment of hyaline cartilage composition, as well as its importance in detecting and monitoring cartilaginous degeneration.
SQ scoring methods for MR imaging assessment of whole knee joint
Assessment based on SQ scoring methods has added greatly to the understanding of the pathophysiology and natural history of knee OA as well as the clinical implications of the structural changes. These techniques allow assessment of articular structures that are currently believed to be relevant to the functional integrity of the knee or that are potentially involved in the pathophysiology of OA in terms of progression and pain. These structural features include articular cartilage morphology, subchondral bone marrow abnormalities, presence of marginal and central osteophytes, meniscal morphology and position, cruciate and collateral ligament integrity, presence of synovitis and effusion, and intra-articular loose bodies, as well as periarticular cysts and bursitis.
Whole-organ assessment scoring of different joint structures on MR imaging has shown adequate reliability, specificity, and sensitivity, as well as the ability to detect lesion progression. Three SQ scoring systems for whole-organ assessment of knee OA have been validated: the whole-organ magnetic resonance imaging score (WORMS), the knee osteoarthritis scoring system (KOSS), and the Boston-Leeds osteoarthritis knee score (BLOKS). Additional scoring tools have been introduced for joint conditions that may not be adequately assessed by the current systems or that offer alternative approaches. Examples are the assessment of synovitis on contrast-enhanced MR imaging or detailed evaluation of the intercondylar tibial region. An overview of the 3 different whole-organ scoring systems is presented in Table 1 .
| BLOKS | KOSS | WORMS | |
|---|---|---|---|
| Number of knees scored in original publication | 10 knees (71 knees for validity exercise of BML scoring) | 25 knees | 19 knees |
| MR imaging protocol of original publication (all publications used 1.5-T systems) | For reliability exercise (10 knees): sag/cor T2w FS, sag T1 SE, axial/cor 3D FLASH For validity of BML assessment: sag PD/T2w Cor/axial PD/T2w FS | Cor/sag T2w and PDw, sag 3D SPGR, axial PD, and axial T2w FS | Axial T1 SE, cor T1 SE, sag T1 SE, sag T2 FS, sag 3D SPGR |
| Subregional division of knee | 9 subregions: medial/lateral patella, medial/lateral trochlea, medial, lateral weight-bearing femur, medial/lateral weight-bearing tibia, subspinous tibia | 9 subregions: medial patella, patellar crest, lateral patella, medial/ lateral trochlea, medial/lateral femoral condyle, medial/lateral tibial plateau | 15 subregions: medial/lateral patella, medial/lateral femur (anterior/central/posterior), medial/lateral tibia (anterior/central/posterior), subspinous tibia |
| Interreader reliability | Performed on 10 knees w-κ between 0.51 (meniscal extrusion) and 0.79 (meniscal tear) | Performed on 25 knees w-κ between 0.57 (osteochondral defects) and 0.88 (bone marrow edema) | Performed on 19 knees ICC between 0.74 (bone marrow abnormalities and synovitis/effusion) and 0.99 (cartilage) |
| Intrareader reliability | Not presented | Performed on 25 knees w-κ between 0.56 (intrasubstance meniscal degeneration) and 0.91 (bone marrow edema and Baker cyst) | Not presented |
| Scored MR Features | |||
| Cartilage | Two different scores Score 1: subregional approach. (A) Percentage of any cartilage loss in subregion; (B) percentage of full-thickness cartilage loss in subregion Score 2: Site-specific approach. Scoring of cartilage thickness at 11 specific locations (not subregions) from 0 (none) to 2 (full-thickness loss) | Subregional approach: focal and diffuse defects are differentiated Depth of lesions is scored from 0 to 3 Diameter of lesion is scored from 0 to 3 Osteochondral defects are scored separately | Subregional approach: scores from 0 to 6 depending on depth and extent of cartilage loss. Intrachondral signal additionally scored as present/absent |
| BMLs | Scoring of individual lesions Three different aspects of BMLs are scored: 1. Size of BML scored from 0 to 3 concerning percentage of subregional bone volume 2. Percentage of surface area adjacent to subchondral plate 3. Percentage of BML that is noncystic | Scoring of individual lesions from 0 to 3 concerning maximum diameter of lesion | Summed BML size/volume for subregion from 0 to 3 in regard to percentage of subregional bone volume |
| Subchondral cysts | Scored together with BMLs | Scoring of individual lesions from 0 to 3 concerning maximum diameter of lesion | Summed cyst size/volume for subregion from 0 to 3 in regard to percentage of subregional bone volume |
| Osteophytes | Scored at 12 sites from 0 to 3 | Scored from 0 to 3 Marginal, intercondylar and central osteophytes are differentiated Locations/sites of osteophyte scoring not forwarded | Scored at 16 sites from 0 to 7 |
| Bone attrition | Not scored | Not scored | Scored in 14 subregions from 0 to 3 |
| Effusion | Scored from 0 to 3 | Scored from 0 to 3 | Scored from 0 to 3 |
| Synovitis | 1. Scoring of size of signal changes in Hoffa fat pad 2. Five additional sites scored as present/absent (details of scoring not described) | Synovial thickening scored as present/absent on sagittal T1w SPGR sequence (location not described) | Combined effusion/synovitis score |
| Meniscal status | Anterior horn, body, posterior horn scored separately in medial/lateral meniscus Presence/absence scored: intrameniscal signal vertical tear horizontal tear complex tear root tear macerated meniscal cyst | No subregional division of meniscus described Presence/absence of following tears horizontal tear vertical tear radial tear complex tear bucket-handle tear meniscal intrasubstance degeneration scored from 0 to 3 | Anterior horn, body, posterior horn scored separately in medial/lateral meniscus from 0 to 4: minor radial or parrot beak tear nondisplaced tear or prior surgical repair displaced tear or partial resection complete maceration/destruction or complete resection |
| Meniscal extrusion | Scored as medial and lateral extrusion on coronal image and anterior extrusion for medial/lateral meniscus on sagittal image from 0 to 3 | Scored on coronal image from 0 to 3 | Not scored |
| Ligaments | Cruciate ligaments scored as normal or complete tear Associated insertional BMLs are scored in tibia and in femur | Not scored | Cruciate ligaments and collateral ligaments scored as intact or torn |
| Periarticular features | Patella tendon: no signal change and signal abnormality The following features are scored as present or absent: Pes anserine bursitis Iliotibial band signal Popliteal cyst Infrapatellar bursa Prepatellar bursa Ganglion cysts of the TFJ, meniscus, ACL, PCL, semimembranosus, semitendinosus, other | Only popliteal cysts scored from 0 to 3 | Popliteal cysts, anserine bursitis, semimembranosus bursa meniscal cyst, infrapatellar bursitis, prepatellar bursitis, tibiofibular cyst scored from 0 to 3 |
| Loose bodies | Scored as absent/present | Not scored | Scored from 0 to 3 |
Several epidemiologic studies and clinical trials have used WORMS, introduced by Peterfy and colleagues, to evaluate OA features of the knee. WORMS uses a complex subregional division of the different knee compartments ( Fig. 1 ). Features assessed by WORMS are: cartilage morphology and signal, subchondral bone marrow edemalike lesions or the synonymous bone marrow lesions (BMLs), subchondral cysts, osteophytes, subchondral bone attrition, meniscal morphology and position, and a combined effusion/synovitis score as well as collateral and cruciate ligaments status. In addition, several periarticular features are evaluated such as meniscal and popliteal cysts, periarticular bursitis, and loose bodies. BMLs and cysts are scored depending on the percentage amount of subregion. Unlike the other systems, WORMS uses a strict subregional rather than a lesion-oriented approach especially to the scoring of cartilage, BMLs, and subchondral cysts. This strategy offers the advantage of summing several lesions per subregion and facilitates assessment and subsequent analyses.

The KOSS system, introduced by Kornaat and colleagues, covers similar MR imaging-detected OA features as WORMS, but with differences: cartilage status, subchondral BMLs, and cysts are scored individually for each subregion and each score is differentiated by lesion size. Osteophytes are differentiated into marginal, intercondylar, and central. Although KOSS uses a more complex meniscal score for tear morphology than WORMS, it does not describe regional subdivisions or partial or total meniscal maceration/resection.
The BLOKS system was published by Hunter and colleagues in 2008. BLOKS uses an approach similar to KOSS in the regional division of the knee joint, focusing on the weight-bearing components versus the patellofemoral joint ( Fig. 2 ). BMLs and cysts are evaluated by taking into account number and size of BMLs, percentage of involved subchondral surface area of the BML and the percentage of BML that is cystic. Thus, subchondral cysts are defined as part of the BML and are not assessed separately as in WORMS and KOSS. The lesional approach for BML assessment allows for superior longitudinal analysis of individual lesions. On the other hand, defining the exact number of individual lesions is sometimes difficult because lesions may be directly adjacent to each other or merge or split in longitudinal assessments. Cartilage scoring takes into account percentage of any cartilage loss in the subregion and percentage of cartilage damage that represents full-thickness loss. Signal changes in the Hoffa fat pad are scored as a surrogate for synovitis. BLOKS uses a complex system to evaluate the meniscal status including different types of tears, intrasubstance signal changes, and meniscal extrusion.

Recent work based on Osteoarthritis Initiative (OAI) data evaluated how differences between WORMS and BLOKS in scoring cartilage, meniscus, and subchondral BMLs affected the assessment of presence, extent, and severity of structural changes of knee OA. The investigators concluded that the ideal MR imaging reading system for OAI data should include elements from both systems. Excellent reliability data have been published for all 3 whole-organ SQ scoring systems ( Table 2 ).
| Joint Feature | WORMS Interreader Agreement (ICC) | KOSS Interreader (ICC [95% CI]/w-κ) | KOSS Intrareader (ICC [95% CI]/w-κ) | BLOKS Interreader (w-κ [95% CI]) |
|---|---|---|---|---|
| BML size | 0.74 | 0.91 [0.88–0.93]/0.88 | 0.93 [0.91–0.94]/0.91 | 0.72 [0.58–0.87] |
| BML % area (BLOKS only) | N/A | N/A | N/A | 0.69 [0.55–0.82] |
| % of lesion BML (BLOKS only) | N/A | N/A | N/A | 0.72 [0.58–0.87] |
| Osteophytes | 0.97 | 0.71 [0.67–0.76]/0.67 | 0.76 [0.72–0.80]/0.79 | 0.65 [0.52–0.77] |
| Cartilage morphology | 0.99 | 0.64 [0.58–0.69]/0.57 | 0.78 [0.74–0.81]/0.67 | 0.72 [0.59–0.85] |
| Cartilage 2 (BLOKS only) | N/A | N/A | N/A | 0.73 [0.60–0.85] |
| Osteochondral defects (KOSS only) | N/A | 0.63 [0.55–0.70]/0.66 | 0.87 [0.83–0.90]/0.87 | N/A |
| Synovitis | 0.74 | 0.74 [0.58–0.85] | 0.81 [0.69–0.89]/0.77 | 0.62 [0.05–1.00] |
| Effusion | See synovitis; scores combined | See synovitis; scores combined | See synovitis; scores combined | 0.61 [0.05–0.85] |
| Meniscal extrusion/ subluxation | N/A | 0.67 [0.57–0.75]/0.65 | 0.82 [0.75–0.86]/0.82 | 0.51 [0.24–0.78] |
| Meniscal signal/intrasubstance degeneration | N/A | 0.78 [0.68–0.85]/0.66 | 0.76 [0.66–0.83]/0.56 | 0.68 [0.44–0.93] |
| Meniscal tear | 0.87 | 0.70 [0.61–0.77]/0.70 | 0.78 [0.70–0.83]/0.78 | 0.79 [0.40–1.00] |
| Ligaments | 1.0 | N/A | N/A | N/A |
| Subchondral cysts | 0.94 | 0.87 [0.83–0.89]/0.83 | 0.90 [0.87–0.92]/0.87 | Part of % BML score |
| Baker cysts | N/A | 0.89 [0.76–0.95]/0.80 | 0.96 [0.90–0.98]/0.91 | N/A |
Several factors have to be considered when deciding which scoring system should be applied for the assessment of a given study. The most important are the outcome measures that are relevant to the study. Second, resources have to be taken into account because assessment using a complete whole-organ score differs from scoring only certain selected features. Last, the image data set is important because not all features are scorable on all sequences or with any given sequence protocol.
No literature is available concerning knee OA to determine whether several consecutive MR imaging examinations from the same patient should be evaluated semiquantitatively with chronologic order known to readers or if the images should be presented with readers blinded to the sequence in which they were acquired. The primary rationale behind blinding to sequence is to reduce reader bias toward change in the expected direction. However, as long as readers are blinded to treatment assignment in a clinical trial it is not necessary to blind them to the chronologic sequence of the image data because bias cannot influence the trial results. If the research aim is not treatment but rather the natural history and detection of change, blinding might be of advantage.
Role of MR imaging-detected structural abnormalities in predicting pain and progression of disease in knee OA
Subchondral BMLs
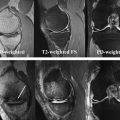
BMLs are defined on MR imaging as noncystic subchondral areas of ill-defined hyperintensity on proton density-weighted, intermediate-weighted, T2-weighted or short tau inversion recovery (STIR) sequences, displaying low signal intensity on T1-weighted spin echo images. MR imaging assessment of BMLs should be performed only on such sequences, because gradient recalled echo (GRE)-type sequences such as spoiled gradient echo at a steady state, fast low-angle shot, 3-point Dixon, double-echo steady state (DESS) and others are insensitive to marrow abnormalities because of trabecular magnetic susceptibility or T2∗ effects, and may lead to underestimation of BML size ( Fig. 3 ). It is important to distinguish degenerative BMLs from other marrow alterations of traumatic or nontraumatic origin, because the differential diagnoses are broad. These degenerative lesions are frequently detected in conjunction with cartilage damage in the same region, along with other OA features such as adjacent osteophytes. Knowing the specific MR imaging characteristics of such lesions is crucial for accurate detection and quantification.
BMLs play an important role in predicting structural progression and pain incidence as well as fluctuation of symptoms in patients with knee OA. The term bone marrow edemalike lesion or the synonymous bone marrow lesion is now widely accepted to describe these alterations, because edema seems to be only a minor constituent of these abnormalities.
Concerning the natural history of these lesions, BMLs represent a highly variable feature in patients with or at risk for development of knee OA, because their size may increase or decrease over time. Mechanical limb alignment is believed to directly affect location, prevalence, and change in BMLs, because medial knee BMLs occur mainly in varus-aligned limbs, and lateral lesions occur mostly in valgus-aligned limbs. Furthermore, these lesions are associated with concomitant increased local bone density, suggesting that they may be secondary to long-term excess loading.
Several studies evaluating the role of BMLs in the progression of knee OA are available. In a longitudinal study assessing the association between BMLs and radiographic progression of knee OA, Felson and colleagues reported that BMLs are powerful predictors of risk of local structural deterioration. Changes in BML size over time seem to have a direct effect on progression of knee OA. In a longitudinal MR imaging-based study, Roemer and colleagues showed that subregions within the knee having incident and progressive BMLs had a higher risk of cartilage loss at follow-up and that absence of BMLs was associated with a lesser risk of cartilage loss in the same subregion. Hunter and colleagues reported in a longitudinal study that, compared with stable BMLs, enlarging lesions were strongly associated with cartilage loss at follow-up. In the same study, presence of BMLs was strongly associated with malalignment and the effect of these lesions on cartilage loss was diluted after adjustment for limb alignment. In a recent longitudinal study, Davies-Tuck and colleagues showed that development of new BMLs was associated with progressive cartilage loss after 2 years, whereas resolution of prevalent BMLs was associated with reduced progression of cartilage loss.
One could argue that the relationship between BMLs and degenerative cartilage lesions in knee OA might be seen as mutually predictive, because both features are highly related cross-sectionally. In a recent longitudinal study of patients with or at risk for knee OA, the investigators considered baseline degenerative cartilage lesions as predictors of BMLs in the same subregion. They found a strong association between prevalent cartilage damage and incident BMLs in the same subregion after adjustment for potential confounders. It seems that subchondral bone and cartilage cannot be assessed and managed separately in OA, and the concept of an osteochondral unit might be fruitful in future OA research.
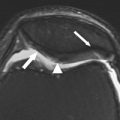
Another type of BML includes those in areas not covered by articular cartilage, such as the interspinous region at the tibia and the femoral notch. These lesions, known as traction or insertional BMLs, are highly associated with cruciate ligament tears, and may be a consequence of tensile stress on these ligaments ( Fig. 4 ). No relationship between lesions at the interspinous region and femoral notch and cartilage loss has been shown. However, lesions at the interspinous region extending to the subchondral bone of the medial tibial plateau are associated with regional cartilage loss.
The role of BMLs in predicting pain in patients with knee OA is controversial. In a cross-sectional study Felson and colleagues reported that patients with radiographic knee OA and pain were more likely to have BMLs than patients without pain. Larger BMLs were found predominantly in the painful group. In a longitudinal study evaluating the relationship between fluctuation of BML size and knee pain, the same group found that individuals without frequent knee pain who developed knee pain at follow-up were more likely to show an increase in BML size. In contrast, Kornaat and colleagues found in a longitudinal study that changes in BMLs did not correlate with severity of pain as measured by WOMAC scores (Western Ontario and McMaster Universities Osteoarthritis Index). Furthermore, patients in whom BML size increased did not have a higher WOMAC score than patients with a decrease in BML size. Sowers and colleagues found that frequency of BMLs was similar in both painful and painless knee OA, but larger BMLs were more frequent in patients with pain.
Subchondral Cystlike Lesions
Subchondral cystlike lesions are a common finding in patients with knee OA. These lesions have a characteristic appearance on MR imaging, showing well-defined rounded areas of fluidlike signal intensity on nonenhanced imaging. The term subchondral cystlike lesion is probably more appropriate than subchondral cyst because no evidence of epithelial lining was detected in several histologic studies. Furthermore, in a recent cross-sectional study of patients with or at risk of knee OA, most of these lesions enhanced on MR imaging after intravenous administration of paramagnetic contrast agent, a feature not expected of pure cystic lesions.
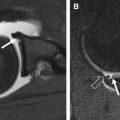
The cause of subchondral cystlike lesions is controversial. Two principal theories have been proposed, the synovial fluid intrusion and the bony contusion theories ( Fig. 5 ). The synovial fluid intrusion theory posits that increased intra-articular pressure may lead to the intrusion of joint fluid into the subchondral bone via fissured or ulcerated cartilage, creating the lesions. The bony contusion theory suggests that subchondral cystlike lesions are a consequence of traumatic bone necrosis after impact of 2 opposing articular surfaces.
A recent cross-sectional MR imaging-based study reported that subchondral cystlike lesions were present in subregions without full-thickness cartilage defects in about half of the cases, which does not support the synovial fluid intrusion theory. Subchondral cystlike lesions are strongly associated with BMLs in the same subregion, and may develop within areas of noncystic BMLs, which favors the bony contusion theory. A recent longitudinal MR imaging-based study assessed the incidence of subchondral cystlike lesions in subregions presenting at baseline with full-thickness cartilage loss (synovial intrusion theory) versus BMLs (bony contusion theory), showing an important association of incident subchondral cystlike lesions with baseline BMLs, even after adjustment for potential confounders, which strongly supports the bony contusion theory of subchondral cyst formation.
Subchondral cystlike lesions do not seem to play a role in predicting knee pain. Two studies have found no association between the presence of subchondral cystlike lesions and pain in patients with knee OA.
Subchondral Bone Attrition
Subchondral bone attrition is defined as depression or flattening of the subchondral bony surface unrelated to gross fracture. It can be assessed on radiographs or semiquantitatively on MR imaging. Although attrition is usually observed in advanced knee OA, it may also appear in knees with mild OA that do not show joint space narrowing on radiographs.
The pathogenesis of subchondral bone attrition in knee OA is unknown. Subchondral microfracturing and remodeling caused by alterations in mechanical loading, which are reflected as subchondral BMLs, may explain the presence and development of bone attrition in OA. A strong association between prevalent subchondral bone attrition and subchondral BMLs in the same subregion has been reported ( Fig. 6 ), and the association increased with BML size. Furthermore, the risk of incident subchondral bone attrition was increased for subregions with baseline BMLs. Neogi and colleagues reported that both prevalence and incidence of subchondral bone attrition are associated with knee malalignment, suggesting that attrition is a reflection of compartment-specific mechanical load, a finding that also approaches BMLs from subchondral attrition. The same group found that subchondral bone attrition is a good predictor of cartilage loss longitudinally.
Subchondral bone attrition seems to play a role in predicting knee pain. In a recent cross-sectional study, Hernandez-Molina and colleagues reported that bone attrition was associated with knee pain in OA, even after adjustment for other known factors linked to pain, suggesting an independent association of these features. Other studies have also suggested that subchondral bone attrition predicts knee pain.
Meniscal Disease
The meniscus plays a critical protective role in the tibiofemoral compartments because of its shock-absorbing and load-distributing properties. The menisci act on transmission of axial and torsional forces across the tibiofemoral joint and distribute mechanical loads over a wider area. Meniscal disease is commonly observed in patients with and without radiographic knee OA. It is rare to find normal meniscal morphology in compartments with OA; instead, the meniscus is often torn, macerated, or even totally destroyed, suggesting a strong association between tibiofemoral OA and meniscal disease. Meniscal tears as well as partial or complete loss of overall normal morphology of the menisci (meniscal maceration/resection) may interfere with its functions and may lead to cartilage loss of the same compartment as well as in the subchondral bone, ultimately contributing to progression of OA. The peak and average contact stresses in the medial compartment increase in a range of 40% to 700% when these functions are lost.
Meniscal pathology plays a role in predicting cartilage loss in the tibiofemoral compartments ( Fig. 7 ). In a recent longitudinal study, Hunter and colleagues showed that displaced meniscal tears and meniscal maceration ( Fig. 8 ) had a higher association with regional cartilage loss than nondisplaced meniscal tears, with the most normal meniscus (without tears or maceration) used as the reference group. Another recent longitudinal study reported that not only maceration but also single horizontal tears in the medial meniscus were associated with cartilage loss in the medial compartment. Horizontal meniscal tears ( Fig. 9 ) are believed to be degenerative lesions, often associated with older age and preexisting or incipient osteoarthritic disease. Thus, horizontal (degenerative) meniscal tears could be an early sign of degeneration of the tibiofemoral compartment, including the underlying articular cartilage. After meniscal tearing and loss of its function, the underlying articular cartilage is less able to withstand the increased loading, and progression of cartilage loss is seen.
Abnormal signal within the substance of the meniscus not touching the articular surface is often detected in middle-aged and elderly patients. These signal changes, often globular or linear in shape ( Fig. 10 ), are believed to represent either intrameniscal degeneration or intrasubstance tear. The role of intrasubstance meniscal alterations in predicting knee pain is controversial, and little is known about their role in progression of cartilage loss in the same compartment of the knee. A recent longitudinal study showed that abnormal intrasubstance medial meniscal signal detected at baseline was not associated with cartilage loss in the medial compartment 24 months later. This finding may indicate that medial meniscal function may be preserved even when such signal alterations are present, although one could argue that follow-up in that study was short.
Meniscal extrusion is commonly seen among middle-aged and elderly patients, especially in compartments with OA. Meniscal extrusion also predicts cartilage loss longitudinally ( Fig. 11 ), because it may increase the contact stress on tibial and femoral articular cartilage, and may also contribute to increased joint space narrowing seen on radiographs. Meniscal tears are considered the main predictor of extrusion, because tearing interrupts the circumferential hoop collagen fiber orientation. A recent cross-sectional study using a large cohort (more than 1000) with or at risk for knee OA showed a strong and significant association between meniscal tears and meniscal extrusion, with a direct relationship between degree of extrusion and severity of meniscal lesion. The same study showed also that not only meniscal tears but also knee malalignment as well as cartilage damage were associated with meniscal extrusion after adjustment for the presence of concomitant meniscal tears in the same compartment. Meniscal tears in OA may be associated with symptoms, but not with most lesions. Pain or discomfort might be present, especially in peripheral tears (red zone) or in dislocated tear fragments.
Synovitis
Synovitis in OA is believed to be a secondary phenomenon related to cartilage deterioration. However, its importance in the OA process is well recognized. Furthermore, degenerative joints usually show signs of synovitis, even in the early phase of disease.
Several nonenhanced and contrast-enhanced MR imaging techniques for detecting and quantifying synovitis are available. In a pathologic study conducted by Fernandez-Madrid and colleagues, MR imaging detected signal alterations in the Hoffa fat pad correlated with mild chronic synovitis. This work led to the assumption that synovitis may be assessed on nonenhanced images, mainly on proton density-weighted or T2-weighted sequences, using signal alterations in the Hoffa fat pad as a surrogate for whole-knee synovitis ( Fig. 12 ). However, signal alterations in the Hoffa fat pad are a common finding on MR imaging of the knee and present many possible diagnoses. Roemer and colleagues found that signal alterations in the Hoffa fat pad seen on noncontrast-enhanced sequences were a sensitive but not a specific sign of peripatellar synovitis, compared with contrast-enhanced sequences. Recently another scoring system for the assessment of synovitis using nonenhanced scans was introduced, but it has not been tested against an established reference standard such as contrast-enhanced MR imaging or histology. In a recent study comparing 3 scoring systems for evaluating synovitis and joint effusion on MR imaging, Loeuille and colleagues found that only scoring of contrast-enhanced T1-weighted images correlated with microscopically proved synovitis. Furthermore, no correlation with microscopic synovitis was found when MR imaging was performed without contrast intravenous administration. Thus, ideally, synovitis should be assessed on contrast-enhanced T1-weighted MR imaging sequences, allowing evaluation of enhancement and thickening of the synovial membrane ( Fig. 13 ). Only contrast-enhanced images can differentiate between synovium and joint effusion. A new scoring system that uses contrast-enhanced T1-weighted sequences to assess synovitis at multiple sites in patients with knee OA was presented recently. Synovial thickness was measured at the peripatellar region, around the cruciate ligaments and menisci, and around popliteal cysts and loose bodies if present, allowing assessment of synovitis in the whole joint. The reliability of the reading was good to excellent for the 11 different synovitis locations. The region around the cruciate ligaments seems to be the most commonly affected, a novel finding of possible clinical relevance in regard to the role of ligament integrity in the OA process.
There is evidence that synovitis also plays a role in progression of cartilage loss in knee OA. In a longitudinal study with 422 patients, Ayral and colleagues assessed the medial perimeniscal synovium and the medial tibiofemoral cartilage using arthroscopy, and found that 123 (29%) patients had a reactive aspect and 89 (21%) had an inflammatory aspect of the synovium. Only the inflammatory synovitis group showed an association with cartilage loss at follow-up. Although histologic evaluation was not performed, previous studies have shown a good correlation between arthroscopic and microscopic findings of synovitis.
Synovial inflammation is believed to contribute to pain in patients with knee OA, even although nociceptive fibers are inconsistently present within the synovial membrane. Hill and colleagues showed that alterations in the Hoffa fat pad signal changes over time were modestly and directly correlated with changes in knee pain, but not with cartilage loss. The effect of these signal alterations on pain was independent of changes in joint effusion. In another cross-sectional study, the same group found that these alterations were more common in patients with knee pain and radiographic OA than those with radiographic OA and no pain. However, both studies relied on noncontrast-enhanced MR imaging sequences. Recent studies assessing synovitis on contrast-enhanced MR imaging in patients with or at risk for knee OA reported that high-grade synovitis (graded semiquantitatively from 0 to 2) was associated with knee pain compared with patients with no or low-grade synovitis.
Effusion
Joint effusion is commonly detected in patients with moderate to advanced knee OA, and reflects synovial activation secondary to ligament injury, loose bodies, hyaline cartilage deterioration, and meniscal damage. Joint effusion is ideally assessed and quantified on proton density-weighted, T2-weighted, and STIR MR imaging sequences. However, synovial thickening as seen in synovitis increases the total synovial volume in such sequences, and differentiating synovium from effusion on nonenhanced MR imaging sequences is often difficult.
The prevalence of joint effusion in knee OA has a direct relationship with radiographic severity in the knee joint. In a cohort of 1368 knees without radiographic knee OA, the prevalence of joint effusion was 33.7% and most effusions were small. Hill and colleagues reported a high prevalence of joint effusion in individuals with radiographic knee OA. Effusion was present in 91.7% of patients with radiographic OA and knee pain, and in 82.3% of those with radiographic OA and no pain. The same study reported that moderate and large effusions (graded from 0 to 3) were significantly more common among those with knee pain. A significant association between grades of effusion (graded in conjunction with synovitis on nonenhanced MR imaging) with knee pain severity was found by Torres and colleagues in a cohort of 143 patients with knee OA. The joint capsule contains pain fibers, and capsule distension associated with joint effusions may contribute to knee pain in OA.
Cruciate and Collateral Ligaments
Traumatic complete anterior cruciate ligament (ACL) tears may lead to premature knee OA. However, the role of traumatic incomplete ACL tears in predicting knee OA is controversial. ACL disruption inevitably causes alterations in knee kinematics, because the ACL is the primary restraint against tibial translation. Furthermore, ACL failure increases the external adduction moment and consequently medial loading, increasing the risk of medial tibiofemoral OA. ACL tears are frequently associated with other traumatic lesions in the knee such as meniscal tears and chondral/osteochondral lesions, making the assessment of their role in knee degeneration more difficult.
Incidental ACL tears are common among patients with knee OA, with reported prevalence ranging from 20% to 35%. Patients with knee OA and incidental ACL tears are often unable to recall significant knee injury. Degeneration within the ligament fibers, alterations in notch width and depth, and the presence of notch osteophytes ( Fig. 14 ) may predispose to ACL tears in patients with knee OA. Evaluation of cruciate ligaments must include not only assessment for tears but also for insertional or traction BMLs. Hernandez-Molina and colleagues showed that traction BMLs, detected on MR imaging at the femoral and tibial insertions of the ACL, are strongly related to ACL disease.
The role of ACL tears in predicting structural progression in patients with knee OA remains unclear. In a recent longitudinal study, Amin and colleagues found that the presence of an ACL tear at baseline increased the risk for cartilage loss in the medial compartment at 30-month follow-up. However, adjustment for medial meniscal damage diluted the effect. In a large cohort of 245 elderly individuals (aged 70–79 years), the prevalence of any ligament tear in the knee was 27% in men and 30% in women, and a good correlation with cartilage loss was found. However, there was no longitudinal assessment.
The contribution of ACL tears to pain severity in patients with knee OA is also unclear. Hill and colleagues showed that complete ACL tears were common (22.8%) in a population with symptomatic knee OA and poor recall of knee trauma, and rare (2.7%) among those without knee symptoms. Another group reported that patients with a complete ACL tear tended to have greater knee pain at baseline, but no overall differences in pain severity were found after adjustment for potential confounders. In both studies, the ACL was scored as pathologic when a complete tear was detected.
The posterior cruciate ligament (PCL) plays a role in the kinematics of the knee, especially for the medial compartment, and a tear with a subsequent PCL deficiency may increase the incidence of knee OA. In a long follow-up study of 58 patients with isolated partial or complete PCL tears treated conservatively and evaluated after 2 to 19.3 years (mean 6.9 years), Patel and colleagues found that 10 (17.2%) developed medial tibiofemoral radiographic OA. Incidental complete PCL tears are rare among patients with knee OA.
Incidental collateral ligament tears are infrequent among patients with knee OA. In a small cohort of 30 patients with medial compartment knee OA with no history of trauma and 30 age-matched patients with atraumatic knee pain but without OA, signal changes in or around the medial collateral ligament (MCL) (grade 1 and 2 lesions) were seen in 27 (90%) of the first group, but in only 2 (6.6%) from the control group, suggesting that grade 1 and 2 MCL lesions may be related to medial knee OA in patients without history of trauma. The role of collateral ligament abnormalities in predicting structural progression and pain in patients with OA is unknown.
Periarticular Cysts and Bursae
A wide spectrum of periarticular cystic lesions may be encountered around the knee in patients with OA. Most cystic lesions around the knee are encapsulated fluid collections showing low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.
Popliteal (Baker) cysts are not true cysts, but fluid in the semimembranosus-medial gastrocnemius bursa, and are commonly detected in patients with knee OA. Hill and colleagues found that the prevalence of these lesions was 43.2% in knees with moderate or larger effusions, compared with 22.7% in those with little or no effusion. In this study, presence of popliteal cysts was not associated with pain. However, different grades of synovitis may be present around popliteal cysts, and such a feature should be assessed on contrast-enhanced MR imaging (see Fig. 13 ). Moderate to large popliteal cysts are associated with incident radiographic knee OA.
A wide spectrum of bursitides may occur in patients with knee OA. Prepatellar bursitis may be seen in conjunction with knee OA, but its pathogenesis is believed not to be directly linked to degeneration. A less common site of bursitis is the superficial infrapatellar bursa, appearing on MR imaging as a fluid collection anterior to the tibial tubercle. A tiny amount of fluid within the deep infrapatellar bursa is frequently detected on MR imaging of the knee, including patients with OA. However, this may be considered a normal finding without clinical significance, because of its high prevalence in asymptomatic patients. Anserine bursitis may be detected in conjunction with knee OA, but its association with degeneration is controversial. Chronic anserine bursitis is believed to be most common in elderly patients with degenerative disease or rheumatoid arthritis. However, a recent case-control study found no association between prevalent anserine bursitis and radiographic knee OA. Furthermore, anserine bursitis shows no significant association with incident knee pain or incident radiographic OA.
Parameniscal cysts are believed to be formed by fluid extravasation through a meniscal tear into the parameniscal soft tissue. Most of these cysts result from horizontal tears, which are believed to be of degenerative origin and are a common finding in knee OA. Lateral meniscal cysts are associated with incident knee pain longitudinally.
Ganglion cysts around the knee are routinely detected on MR imaging examinations. They may be seen in conjunction with OA, but accepted theories for ganglia formation are not directly related to OA. Tibiofibular synovial cysts are more prevalent in patients with knee effusion, because in 10% of adults the proximal tibiofibular joint communicates with the knee joint. The reported prevalence in patients with knee OA is low. Other cystic lesions around the osteoarthritic knee are rare.
Loose Bodies
Loose bodies are frequently detected in conjunction with knee OA on MR imaging, especially in severe cases. Loose bodies are a catchall term that may include, for example, chondral fragments, detached osteophytes, and meniscal fragments. Synovial osteochondromatosis secondary to OA should also be considered. Loose bodies are related to internal knee derangement in patients with OA, because they may trigger synovial inflammation as shown by a recent study using contrast-enhanced MR imaging, and are a common indication for arthroscopic treatment. On MR imaging, loose bodies are best visualized in joints with prevalent effusion and may be delineated as solitary or multiple low signal intensity abnormalities within the joint ( Fig. 15 ).