Anorectal malformation (ARM) occurs in approximately 1 in 5000 newborns and is frequently accompanied by anomalies of the genitalia, gynecologic system, urinary tract, spine, and skeletal system. Diagnostic imaging plays a central role in ARM evaluation. Because of the lack of ionizing radiation, excellent intrinsic contrast resolution, multiplanar imaging capabilities, technical advances in hardware, and innovative imaging protocols, magnetic resonance (MR) imaging is increasingly important in assessment of ARM patients in utero, postnatally before definitive surgical correction, and in the postoperative period. This article discusses the role of MR imaging in evaluating ARM patients.
Key points
- •
Anorectal malformation (ARM) comprises a diverse spectrum of congenital malformations of the anus and rectum with an incidence of approximately 1 in 5000 newborns.
- •
Fetal MR imaging plays an increasingly important role for ARM patients; it helps in providing appropriate parental counseling and enables proper antenatal and postnatal management.
- •
With the advent of faster imaging sequences and more advanced imaging techniques, MR imaging is increasingly being relied on to aid definitive surgical correction planning.
- •
MR imaging is increasingly being called on to aid in the evaluation of postoperative complications following the original corrective surgery in ARM patients.
- •
MR imaging has the potential to serve as an ionizing radiation-free, one-stop shop for the imaging evaluation of ARM patients
Introduction
Anorectal malformation (ARM) comprises a diverse spectrum of congenital malformations of the anus and rectum. These malformations can range in severity from minor and easily treated with excellent prognosis, such as rectoperineal or rectovestibular fistulae, to those that are complex and difficult to manage with relatively poor prognosis, such as cloacal malformation and caudal regression syndrome. Overall, congenital ARM affects approximately 1 in 5000 newborns, with a slight male predominance. The incidence of cloacal malformations has frequently been reported in the literature as approximately 1 in 40,000 to 50,000 newborns, although the incidence is likely more frequent (approximately 1 in 20,000) because many of these patients were previously erroneously diagnosed with a rectovaginal fistula.
Anomalies of the gastrointestinal, genitourinary, skeletal, nervous, and cardiovascular systems are frequently associated with ARM.
Associated anomalies are frequently found in patients with congenital ARM. Although all organ systems can be affected, abnormalities of the gastrointestinal, genitourinary, skeletal, nervous, and cardiovascular systems are most common. Anomalies of the gastrointestinal tract outside of the primary ARM include esophageal atresia and duodenal atresia. Anomalies of the genitourinary tract include but are not limited to absent, dysplastic, or horseshoe kidney; hypospadias; bifid scrotum; vesicoureteral reflux and hydroureteronephrosis; undescended testes; vaginal abnormalities; and Müllerian structure anomalies. Common skeletal abnormalities include sacral agenesis or dysgenesis, spinal dysraphism, vertebral segmentation and fusion anomalies, and scoliosis. Specific spine abnormalities encountered in ARM patients include tethered spinal cord, meningoceles and myelomeningoceles, intradural lipomas, and diastematomyelia. Cardiovascular anomalies are present in 12% to 22% of ARM patients, with tetralogy of Fallot and ventricular septal defect most commonly encountered.
Three specific associations typically encountered with congenital ARM are the Currarino triad; caudal regression syndrome; and the syndrome of vertebral defects, anorectal anomalies or atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb defects (VACTERL). In 1971, Currarino and colleagues first described the triad of ARM, partial agenesis of the sacrum (typically a sickle-shaped sacrum), and presacral mass (typically a teratoma or anterior meningocele). The triad is inherited in an autosomal dominant pattern secondary to a mutation in the HLXB9 homeobox gene.
Caudal regression syndrome is a rare disorder that affects the lower half of the body to varying degrees, including the lower extremities, low back or spine, genitourinary tract, and gastrointestinal tract, including ARM (typically imperforate anus). Approximately 15% to 25% of cases of caudal regression syndrome occur in children of a diabetic mother.
VACTERL is a nonrandom cluster of a group of congenital anomalies. Typically, at least three malformations are required to be diagnosed with the association. The VACTERL association occurs in approximately 1 in 10,000 to 1 in 40,000 newborns, with approximately 55% to 90% of these patients having an ARM.
Currarino triad = ARM, partial agenesis of the sacrum, and presacral mass.
VACTERL = vertebral defects, anorectal anomalies, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb defects.
As with most complex congenital malformations, imaging has long played a critical role in the evaluation and management of patients with ARM. From the first invertogram radiograph described by Wangensteen and Rice in 1930, to contrast fluoroscopic examinations such as contrast enemas, distal high-pressure colostograms, and voiding cystourethrograms, to cross-sectional modalities of today such as ultrasound, CT, and (most recently) MR imaging, diagnostic imaging helps provide the surgeon with the information needed to correct the malformation. To accomplish a successful postoperative outcome, an accurate preoperative imaging assessment is required. This includes assessment of the level and type of malformation, the presence of a fistula, the developmental state of the sphincter muscle complex, and the presence of associated anomalies. In the postoperative patient, accurate imaging is required for identification of postoperative complications, potential reoperative planning, other associated anomalies that may have initially been inconspicuous, and predicting morbidity and quality of life. MR imaging is ideally suited to fulfill these requirements because of its lack of ionizing radiation, excellent intrinsic contrast resolution, and multiplanar imaging capabilities. Disadvantages of MR imaging in ARM patients include the frequent need for sedation, relative high cost, and relative lack of expertise and access to the technique. Despite these disadvantages, MR imaging is increasingly being used by radiologists in the diagnostic work-up of ARM patients, including in the prenatal state, before definitive surgical repair, and postoperatively.
Introduction
Anorectal malformation (ARM) comprises a diverse spectrum of congenital malformations of the anus and rectum. These malformations can range in severity from minor and easily treated with excellent prognosis, such as rectoperineal or rectovestibular fistulae, to those that are complex and difficult to manage with relatively poor prognosis, such as cloacal malformation and caudal regression syndrome. Overall, congenital ARM affects approximately 1 in 5000 newborns, with a slight male predominance. The incidence of cloacal malformations has frequently been reported in the literature as approximately 1 in 40,000 to 50,000 newborns, although the incidence is likely more frequent (approximately 1 in 20,000) because many of these patients were previously erroneously diagnosed with a rectovaginal fistula.
Anomalies of the gastrointestinal, genitourinary, skeletal, nervous, and cardiovascular systems are frequently associated with ARM.
Associated anomalies are frequently found in patients with congenital ARM. Although all organ systems can be affected, abnormalities of the gastrointestinal, genitourinary, skeletal, nervous, and cardiovascular systems are most common. Anomalies of the gastrointestinal tract outside of the primary ARM include esophageal atresia and duodenal atresia. Anomalies of the genitourinary tract include but are not limited to absent, dysplastic, or horseshoe kidney; hypospadias; bifid scrotum; vesicoureteral reflux and hydroureteronephrosis; undescended testes; vaginal abnormalities; and Müllerian structure anomalies. Common skeletal abnormalities include sacral agenesis or dysgenesis, spinal dysraphism, vertebral segmentation and fusion anomalies, and scoliosis. Specific spine abnormalities encountered in ARM patients include tethered spinal cord, meningoceles and myelomeningoceles, intradural lipomas, and diastematomyelia. Cardiovascular anomalies are present in 12% to 22% of ARM patients, with tetralogy of Fallot and ventricular septal defect most commonly encountered.
Three specific associations typically encountered with congenital ARM are the Currarino triad; caudal regression syndrome; and the syndrome of vertebral defects, anorectal anomalies or atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb defects (VACTERL). In 1971, Currarino and colleagues first described the triad of ARM, partial agenesis of the sacrum (typically a sickle-shaped sacrum), and presacral mass (typically a teratoma or anterior meningocele). The triad is inherited in an autosomal dominant pattern secondary to a mutation in the HLXB9 homeobox gene.
Caudal regression syndrome is a rare disorder that affects the lower half of the body to varying degrees, including the lower extremities, low back or spine, genitourinary tract, and gastrointestinal tract, including ARM (typically imperforate anus). Approximately 15% to 25% of cases of caudal regression syndrome occur in children of a diabetic mother.
VACTERL is a nonrandom cluster of a group of congenital anomalies. Typically, at least three malformations are required to be diagnosed with the association. The VACTERL association occurs in approximately 1 in 10,000 to 1 in 40,000 newborns, with approximately 55% to 90% of these patients having an ARM.
Currarino triad = ARM, partial agenesis of the sacrum, and presacral mass.
VACTERL = vertebral defects, anorectal anomalies, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb defects.
As with most complex congenital malformations, imaging has long played a critical role in the evaluation and management of patients with ARM. From the first invertogram radiograph described by Wangensteen and Rice in 1930, to contrast fluoroscopic examinations such as contrast enemas, distal high-pressure colostograms, and voiding cystourethrograms, to cross-sectional modalities of today such as ultrasound, CT, and (most recently) MR imaging, diagnostic imaging helps provide the surgeon with the information needed to correct the malformation. To accomplish a successful postoperative outcome, an accurate preoperative imaging assessment is required. This includes assessment of the level and type of malformation, the presence of a fistula, the developmental state of the sphincter muscle complex, and the presence of associated anomalies. In the postoperative patient, accurate imaging is required for identification of postoperative complications, potential reoperative planning, other associated anomalies that may have initially been inconspicuous, and predicting morbidity and quality of life. MR imaging is ideally suited to fulfill these requirements because of its lack of ionizing radiation, excellent intrinsic contrast resolution, and multiplanar imaging capabilities. Disadvantages of MR imaging in ARM patients include the frequent need for sedation, relative high cost, and relative lack of expertise and access to the technique. Despite these disadvantages, MR imaging is increasingly being used by radiologists in the diagnostic work-up of ARM patients, including in the prenatal state, before definitive surgical repair, and postoperatively.
Embryology, classification, and anatomy
A basic understanding of the normal and pathologic embryology and anatomy of the anorectum, particularly the sphincter mechanism, is helpful when interpreting MR imaging studies in the ARM patient ( Fig. 1 ). The process of normal and abnormal development of the hindgut is not fully understood, although various theories have been offered over the years. Cranially, the hindgut is in continuity with the midgut; caudally, it is in direct contact with the ectoderm, thus forming the cloacal membrane. When development progresses, the caudal part of the hindgut, the cloaca, differentiates into two separate organ systems, the urogenital tract and the anorectal tract. Normal anorectal and genitourinary development depends on the normal development of the dorsal cloaca and the cloacal membrane, the latter having a crucial role in the pathogenesis of ARM. Cloacal membrane defects are thought to also affect development of the genitourinary system and mesenchymal tissue leading to genital malformation and abnormal pelvic floor and sphincter muscle development.
The rectum is the terminal end of the colon, beginning anterior to the level of the third sacral vertebra and extending to the anal canal. The anal canal extends from the anorectal junction to the anal verge. In normal individuals, the muscle groups of the anal sphincter mechanism include the voluntary, striated muscles of the external sphincter and the levator musculature, and the involuntary, smooth muscle internal sphincter. The levator ani muscle is a series of striated muscle groups composed of ischiococcygeus, iliococcygeus, pubococcygeus, and puborectalis. These muscles are continuous with each other. The levator ani muscles extend from the pubic bone, the lowest portion of the sacrum, and the middle of the pelvis downward and medial to join with the external sphincter. The confluence of muscles forms a funneled appearance on coronal imaging planes (see Fig. 1 D). For all practical purposes, no anatomic distinction between the individual muscles of the levator ani or the external anal sphincter can be routinely discerned by MR imaging and their distinction is of no clinical value in the setting of ARM correction. During perineal reconstruction, the surgeon places the mobilized rectum within this muscular funnel, ensuring its correct trajectory through the levator ani, and tacks it to the posterior edge of the muscle complex to end at the neoanus.
No anatomic distinction between the individual muscles of the levator ani or the external anal sphincter can be routinely discerned by MR imaging, and their distinction is of no clinical value in the setting of ARM correction.
Traditionally, ARM patients were classified into high, intermediate, and low malformations. However, this classification system has been found to be arbitrary and somewhat inaccurate. A more practical classification scheme is one that groups ARMs that share common diagnostic, therapeutic, and prognostic features as proposed by Levitt and Peña ( Table 1 ).
| Male | Female |
|---|---|
| Rectovesical (bladder neck) fistula | Rectoperineal fistula |
| Rectourethral (prostatic) fistula | Rectovestibular fistula |
| Rectourethral (bulbar) fistula | Cloaca with short common channel (<3 cm) |
| Rectoperineal fistula | Cloaca with long common channel (>3 cm) |
| Imperforate anus without fistula | Imperforate anus without fistula |
| Rectal atresia | Rectal atresia |
MR imaging in the prenatal period
The ability to identify and characterize ARM on prenatal imaging is important because it allows for appropriate parental counseling, helps to determine the delivery site and plan, and helps the surgeon to plan antenatal and postnatal management. Diagnosing ARM via prenatal ultrasound is challenging. It can be suggested when a fluid-filled, distended rectum is seen in conjunction with a cystic pelvic structure in a female fetus. Even when this combination is seen, typically a wide differential diagnosis must be offered including cystic sacrococcygeal teratoma, anterior meningocele or myelomeningocele, and megacystis-microcolon.
Fetal MR imaging has the potential to diagnose ARM more accurately and with increased confidence relative to ultrasound. As fetal MR imaging availability and expertise becomes more widespread, it will assuredly continue to play an increasing role in the evaluation of suspected ARMs detected during screening prenatal ultrasound.
What the Referring Physician Needs to Know
The referring physician needs to know
- •
Presence of a cystic pelvis mass in a female fetus suggesting a cloacal malformation
- •
Presence of signs indicating mixture of urine and meconium
- •
Presence of associated anomalies of the spine, gastrointestinal, genitourinary, and skeletal systems
Imaging Protocol
Fetal MR imaging for ARM is typically performed in the third trimester of pregnancy. It is important that it not be performed before 20 weeks gestational age because, before this time, the distribution of meconium cannot be accurately defined within the colon and rectum. Fetal MR imaging studies most commonly are performed in a 1.5-T scanner using a phased-array body coil. Neither fetal nor maternal sedation are required. The following sequences are typically obtained in all three planes (relative to the fetus) without fat saturation: (1) T2-weighted single-shot fast spin-echo, (2) two-dimensional (2D) balanced steady-state free precession (bSSFP), and (3) T1-weighted fast gradient recalled echo.
Fetal MR imaging in ARM patients should be performed after approximately 20 weeks gestation. Before this time, abnormal meconium distribution cannot be accurately defined.
Imaging Findings
The normal appearance of the distal colon and rectal cul-de-sac on fetal MR imaging ( Fig. 2 ) has been well described. The rectum should always be identifiable; it should be closely apposed to the bladder regardless of gender, and it should extend at least 10 mm below the bladder neck (increases from 10 to 23 mm with increasing gestation age after 20 weeks). The intraluminal contents of the distal colon and rectum should be hypointense on T2-weighted images, intermediate signal intensity on bSSFP images, and hyperintense on T1-weighted images. The normal maximum distal colon diameter increases with gestational age from approximately 8 mm at 24 weeks to approximately 16 to 18 mm by 35 to 38 weeks.
Normal fetal MR imaging features of the rectum include close apposition to the bladder, extension at least 10 mm below the bladder neck, and meconium signal intensity characteristics.
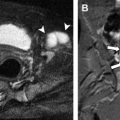
Prenatal gastrointestinal MR imaging findings that have been described with ARM include dilation of the distal colon and rectum ( Fig. 3 A ), abnormal fluid (hyperintense on T2-weighted images) signal within the distal colon and rectum when a fistula is present (see Fig. 3 A), normal signal within the distal colon and rectum when a fistula is not present, separation of the rectum from the posterior bladder wall (see Fig. 3 B), abnormally high location of the rectum relative to the bladder base ( Fig. 4 ), and enterolithiasis ( Fig. 5 ) as the result of long-term mixture of urine and meconium. Abnormalities of the genitourinary system are frequently encountered on prenatal MR images in patients with suspected ARM. These abnormalities include (1) abnormal bladder shape and size; (2) abnormal bladder contents; (3) bladder wall thickening; (4) renal anomalies such as agenesis, hydroureteronephrosis (see Fig. 5 C; Fig. 6 A ), cystic dysplasia (see Fig. 5 C), and multicystic dysplastic kidney; (5) hydrocolpos (see Fig. 6 B); (6) presence of a common channel in cloaca patients; and (7) abnormal external genitalia. Close attention should be paid to potential abnormalities outside of the gastrointestinal and genitourinary systems as well. In two out of four ARM patients identified by prenatal MR imaging by Veyrac and colleagues, and in one out of six ARM patients identified by Calvo-Garcia and colleagues, VACTERL syndrome features were present. Additionally, oligohydramnios may frequently be present on prenatal MR images in ARM patients (see Fig. 6 B).
MR imaging before definitive surgical correction
The postnatal management of children with ARM is predicated on accurate determination of the level and type of malformation as well as the presence and type of fistula. Sometimes, in patients with an imperforate anus and a rectoperineal or rectovestibular fistula, a primary perineal anoplasty can be performed. In most other malformation types, a diverting colostomy is performed in the first few days of life. This is followed by definitive surgical repair (posterior sagittal anorectoplasty [PSARP] with or without laparoscopy depending on the height of the rectum) later in infancy.
Imaging of the newborn with ARM before surgical correction historically relied on invertograms or cross-table lateral radiographs to determine the height of the rectal air if no fistula was evident clinically. Voiding cystourethrograms, ultrasound of the kidneys or bladder and spine, and MR imaging of the spine are mainstays in the imaging work-up of ARM patients. High-pressure distal colostogram remains the gold-standard imaging study for determining the precise anatomy of the distal rectum. The hydrostatic pressure used fully distends the distal rectum by overcoming the compression by the pelvic muscles, thus demonstrating the anatomy accurately and helping the surgeon plan the repair. These imaging examinations, in combination with meticulous clinical assessment and endoscopic evaluation, provide adequate information to plan the operative approach and technique.
The use of preoperative pelvic MR imaging was traditionally reserved for complex ARM or cases in which radiographic, fluoroscopic, and/or ultrasonographic findings were inconclusive. In 1988, Sato and colleagues first reported the MR imaging findings on seven preoperative ARM patients, and correctly identified the level of rectal atresia. Since that time, there have been other infrequent reports of the use of MR imaging in preoperative ARM patients. However, with the advent of faster imaging sequences and more advanced imaging techniques, MR imaging is more frequently being relied on to aid in surgical planning. Techniques such as three-dimensional (3D) MR image reconstruction, MR cloacagram-genitography, and intraoperative MR-guided surgery have been described in the literature. Even though Aslam and colleagues concluded that “MRI has no role as a primary investigation in patients with high ARA [anorectal anomalies],” the authors believe that the advances in MR imaging technology, innovative MR imaging protocols, refinements in surgical techniques, and better understanding of surgical anatomy and clinical outcomes over the past 15 years since that report will result in the continued increase in the use of MR imaging before definitive surgical correction.
MR genitography-cloacography is an MR imaging examination of the pelvis in which dilute gadolinium is instilled into the bladder, vagina, and distal colon rectum (depending on anatomy) to better depict the complex anatomy in ARM patients.
What the Referring Physician Needs to Know
The referring physician needs know
- •
Distal most extent of the fully distended rectum
- •
Presence and location of fistula
- •
Distance from the end of the rectum to the anal dimple
- •
Presence of a presacral mass and other associated congenital anomalies
Imaging Protocol
The ability of 3D reconstructions from preoperative standard MR images to improve understanding of the complex anatomic relationships in ARM patients was first shown in a rudimentary fashion by Ueno and colleagues in 1995, and later by Tang and colleagues using modern MR imaging equipment and sequences. In 2007, Baughman and colleagues, taking advantage of these 3D reconstruction techniques, first described what they termed MR genitography. They concluded that the studies provided excellent anatomic detail and complemented the information obtained by standard pelvic MR imaging and endoscopy in complex ARM patients. Their protocol was performed on a 1.5 T magnet and consisted of routine multiplanar high-resolution T1-weighted and T2-weighted sequences through the pelvis, followed by a 3D T1-weighted spoiled gradient recalled echo (SPGR) sequence with fat saturation acquired during active hand-instillation of dilute gadolinium through balloon catheters via the common channel, mucous fistula, and cutaneous vesicostomy (when present). The gadolinium was diluted with normal saline to a ratio of between 1:750 and 1:1000. Maximum intensity projection (MIP) and 3D volume-rendered reconstructions were performed from the SPGR data set. Coronal screening imaging of the kidneys was also performed. The investigators described a total scanning duration of approximately 30 minutes, and a total patient and scan preparation time of 30 to 60 minutes.
In 2012, Jarboe and colleagues described a similar technique in which they performed MR imaging in combination with 3D rotational fluoroscopy in the same anesthetic setting. The investigators concluded that the combined fluoroscopic and MR imaging cloacagram procedure provided exceptional anatomic definition and aided with surgical planning. Their protocol was performed on a 3 T magnet, and consisted of multiplanar routine T1-weighted and T2-weighted sequences, followed by a 3D T1-weighted SPGR sequence with fat saturation performed after the instillation of a dilute gadolinium solution through balloon catheters and non–balloon catheters via combinations of the mucous fistula, common channel, urinary bladder, vagina, and rectum (depending on specific patient anatomy). Because these studies were performed in combination with a fluoroscopic evaluation of the ARM, the gadolinium was diluted with iodinated contrast in a 1:200 ratio. A vitamin E capsule was placed at the site where the anus should be located to aid in MR image interpretation. 3D volume-rendered and MIP reconstructions were then created from the SPGR data set. At Cincinnati Children’s Hospital Medical Center, these studies are currently performed in a similar fashion but with a more dilute gadolinium concentration of 1:600 because this concentration results in optimal contrast signal intensity. In addition, a coronal fast inversion recovery sequence is added to screen the kidneys. In many cases, screening imaging of the spine is also performed at this same sitting.
Raschbaum and colleagues described the innovative use of intraoperative MR imaging in the performance of laparoscopically-assisted anorectoplasty (LAARP) in three patients with ARM, though the technique has not been widely adopted. One limitation of LAARP is the inability to visualize the narrow path of the vertical muscle fiber complex between the pelvic floor and perineal parasagittal muscle fibers, and the resultant potential for a noncentered pull-through of the rectum to the perineum. By combining intraoperative MR imaging with serial advancement of an MR-compatible needle through the central portion of the vertical muscle fiber complex (as determined with direct muscle stimulation) until the pelvic floor is penetrated, the surgeon is able to identify the needle in the peritoneal cavity and use it to ensure a centered pull-through ( Fig. 7 ).