Fig. 1
CMR provides an excellent modality for delineating cardiac anatomy and function. (a) represents a four-chamber view of a normal heart in diastole and (b) in systole. (c, d) represent images in diastole and systole from a patient with ischemic cardiomyopathy and impaired ejection fraction
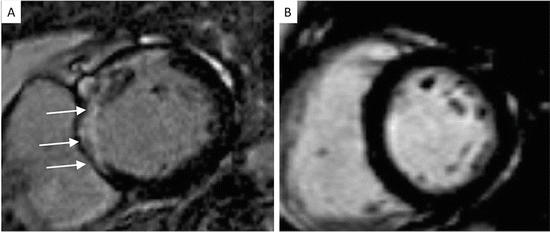
Fig. 2
Following administration of the paramagnetic contrast agent containing gadolinium, areas of myocardial enhancement (white area shown by white arrows) can be identified representing myocardial fibrosis (scarring). Such areas represent not viable myocardium, which will not benefit from revascularization of the coronary artery supplying blood to this area. Image (a) is from a patient with 3-vessel CAD. The area supplied by the left anterior descending (LAD) coronary artery is not viable (white arrows), therefore LAD revascularization will not be beneficial. Image (b) is from a patient with 3-vessel CAD as well where there is no late Gadolinium enhancement (LGE). The myocardium is viable and revascularization can be recommended
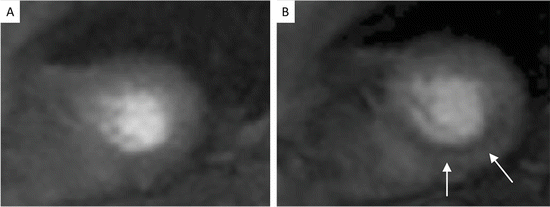
Fig. 3
Images taken from the same patient at rest (a, on the left) and following stress using adenosine (b, on the right). The black area on the right represents a perfusion abnormality indicating reversible ischemia in the right coronary artery (RCA) territory
This early identification allows guiding of pharmacotherapy at a very early stage, offering the opportunity of halting the rate of progression of atherosclerosis and even potentially reversing its burden [11]. Hence, in addition to the relevant anatomical and functional information which can be acquired in a single, safe, noninvasive, and radiation-free examination, CMR has the potential of visualizing the coronary artery lumen and wall to identify early signs of atherosclerosis. However, there are challenges in imaging the coronary artery lumen and wall, limiting the use of CMR in clinical practice at present.
Challenges in Undertaking Coronary Magnetic Resonance Angiography
Since the first studies on the use of coronary MRA (CMRA) [12–15] there have been significant technical advancements, leading to better imaging quality with reduced scanning time. However, movement of the coronary arteries due to cardiac and respiratory motion introduces motion artifacts. Reduction of these artifacts is crucial due to the small and tortuous caliber of the coronary vessels. The following section describes some of the challenges associated with coronary MRA.
Cardiac Motion: Coronary Movement in Systole and Diastole
An ideal subject for visualization using magnetic resonance is something that remains still during the entire acquisition time. In humans for example the upper and lower limbs can be very still and allow for good visualization. The coronary arteries are small, often tortuous, and they are displaced during the cardiac cycle. This leads to significant motion artifacts when trying to visualize the coronaries and the vessel wall. For optimal results, any CMR sequences aimed at visualizing the heart and the coronaries in particular need to take this into consideration. Synchronizing image acquisition to the ECG (R-wave) corresponding to the times of minimal displacement is therefore mandatory for successful coronary artery imaging with CMR. Coronary artery motion is minimal during end-systole and mid-diastole and previous research has shown the advantage of acquiring image data during both rest periods [16]. The specific advantages of both techniques are illustrated in Table 1.
Table 1
Comparison of cardiac imaging in end-systole and mid-diastole in an attempt to image the heart in minimal displacement during the cardiac cycle
Comparison of cardiac imaging with cardiovascular magnetic resonance aiming for minimal displacement | |
|---|---|
During end-systolic rest | During mid-diastolic rest |
Advantages | Advantages |
Heart rate variability less important | Longer acquisition window |
Larger diameter of the venous vessels (if this information required) | Higher signal when gradient echo sequences used |
Disadvantages | Disadvantages |
Shorter acquisition window | Heart rate variability has higher potential to affect image quality |
The optimal trigger delay and the length of the acquisition window vary with the patient’s heart rate. The rest period should be identified for each patient from a high-temporal resolution cine scan usually as a four chamber view performed shortly before the coronary scan [17]. Real time arrhythmia rejection algorithms that facilitate only the inclusion of regular heartbeats within certain limits might further improve image quality for MRA angiography [18, 19].
Respiratory Motion
Another major problem for cardiac imaging with CMR is the displacement of the heart during respiration. It can often reach a distance that can be ten-fold higher than the coronary artery diameter. Synchronization of the image acquisition with the respiratory cycle is therefore mandatory. Several methods have been used to minimize this respiratory motion. Initial approaches for visualizing the proximal native coronary arteries used two-dimensional breath-holding or averaging techniques and mid- or late-diastolic image acquisition with average 6–8 phase encoding lines acquired during each cardiac cycle. However, the main drawback of such techniques was that a long breath-hold of up to 20 s was required for each image slice, which is not easily achieved by every patient. Furthermore, even if patients can initially manage a 20 s breath-hold to complete the MRA an average of 25 slices are required per patient. This can be very tiring and it is therefore common to see the quality of breath-holding decreasing towards the end of the examination, leading to misregistration artifacts between successive breath-holds, degrading the overall image quality. Currently breath-holds for high-resolution three-dimensional datasets are too long and imaging results were rather disappointing. Currently a prospective real-time navigator of the diaphragm is most commonly used and successful technique for coronary lumen and vessel wall imaging.
To compensate in part for this problem, Ehman and Felmee [20] first proposed the use of respiratory MR navigators to remove the time constraint of a breath-hold. The MR navigator (typically an one-dimension pencil beam) is usually placed on the dome of the right hemidiaphragm [21] (Fig. 4). Data are only accepted if the diaphragm-lung interface is within a user-defined window (usually up to 8 mm), preferably positioned around the end-expiratory interface position. If the navigator signal falls outside this predefined window, data is rejected and needs to be reacquired in the following cycle. This results generally in a gating efficiency of around 40–60 %, which means that a large proportion of the scanning time is actually not used [22, 23]. To improve this shortcoming several new self gating navigator approaches have been developed to
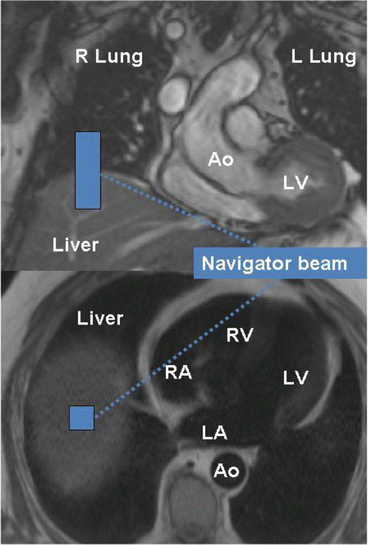

Fig. 4
Using scout images the position of the respiratory navigator can be planned. A pencil beam (one-dimensional navigator) is usually placed on the dome of the right hemi-diaphragm (heart in the left chest). Using the respiratory navigator a three-dimensional volume covering the whole heart can be acquired in a free breathing subject. R Lung right lung, L Lung left lung, Ao aorta, LV left ventricle, RV right ventricle, LA left atrium, RA right atrium
1.
Directly measure the motion of the heart.
2.
Account for the 3D motion of the heart (food-head, left-right, and posterior-anterior).
3.
Achieve 100 % scan efficiency by applying more sophisticated motion correction methods including 3D rigid body or 3D affine.
Cardiovascular Magnetic Resonance Sequences
Image quality for coronary MRA was significantly improved using two-dimensional gradient echo techniques (Edelman [26] and Manning [27]). Sixteen heartbeats were required initially in order to acquire one slice during a single breath-hold, which meant that most patients would tire towards the end of the test. Following the introduction of navigator techniques as described above, three-dimensional imaging covering the whole heart became feasible. Due to its ease to use, this is now the preferred approach in the clinical arena. Several modifications are currently available. The most widely accepted is a T2 preparation technique in combination with a three-dimensional gradient echo (at 3 T) or steady-state free-precession (SSFP) technique (at 1.5 T). Steady-state free-precession sequences are usually preferred at 1.5 T. Although possible at 3 T, the use of SSFP sequences is currently limited by the prolongation of repetition times, increased sensitivity of off-resonance and the need for higher flip angles. T2-preparation and fat suppression techniques are now preferred for noncontrast-enhanced imaging of the coronary artery lumen. The epicardial fat can cause artifacts and fat suppression techniques reduce the signal generated by the epicardial fat tissue, making the lumen of the coronaries more visible [26, 28]. T2-preparation techniques improve the contrast between the coronary lumen and the underlying myocardium [29] as although blood and myocardium have similar T1, they have different T2. Additionally T2-preparation suppresses deoxygenated venous blood leading to better visualization of coronary arteries. An example of the currently most commonly used technique is shown in Fig. 5.
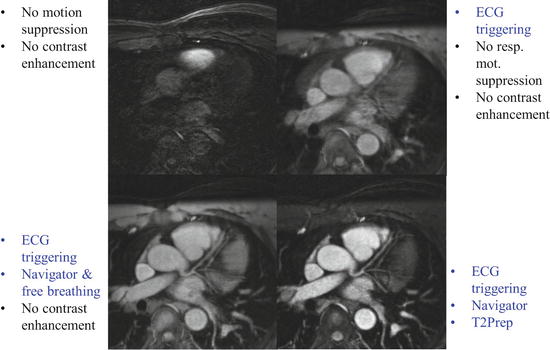

Fig. 5
Progressive improvement in the image quality can be noticed by adding ECG triggering, then a respiratory navigator followed by T2 preparation. (Image courtesy of Matthias Stuber, Professor and Director CVMR/CIBM/CHUV, University of Lausanne, Switzerland)
Contrast Agents for Coronary Angiography
The currently most commonly used type of contrast agents in CMR are nonspecific and distribute in the extracellular space. These are used for first pass contrast-enhanced angiography, perfusion imaging, and late gadolinium enhancement (LGE) imaging of the myocardium [30]. Other types of contrast agents include intravascular targeted [31] and blood pool iron contrast agents (fast and slow blood pool clearance). Each contrast agents has specific characteristics, which needs to be encountered for its clinical use. It is very important to choose the sequence design appropriately for the clinical question and the contrast agent used [31].
On a practical level the ultimate decision depends on the clinical question for the individual patient, balancing the luminographic information required against the vessel wall and myocardial information required. For example, extracellular contrast agents quickly extravasate into the interstitial space, and while they provide excellent detection of scar tissue by LGE (indicating previous myocardial infarction, Fig. 2a), diffuse fibrosis by T1 mapping and viability of the myocardium (suggesting that the muscle is still alive and that myocardial function is likely to improve following revascularization, Fig. 2b), they are suboptimal for coronary artery lumen imaging due to their rapid clearance. Blood-pool contrast agents on the other hand, offer the highest contrast between the vessel lumen and the surrounding tissues but might provide less information regarding late Gadolinium enhancement (LGE). Contrast agents with low albumin-binding properties have the potential for combined coronary artery and infarct imaging This is due to their prolonged retention time in blood and their higher relaxivities thereby improving coronary artery imaging while maintaining a good late enhancement for fibrosis and viability assessment [32]. Intravascular contrast agents are best used for coronary artery lumen imaging with an inversion prepulse instead of a T2-preparation prepulse [33, 34] The diagnostic value of this approach in patients with coronary artery disease still needs to be evaluated in larger clinical studies.
Are We Ready for Coronary Magnetic Resonance Angiography to Enter Routine Clinical Use?
The advances in coronary lumen and vessel wall imaging currently allow clinical applications in selected patient groups [35] (Figs. 6–8). This includes patients with Kawasaki disease [36–38] and imaging the origins and course of the coronary vessels in patients of different age groups for diagnosis and planning of interventional procedures.
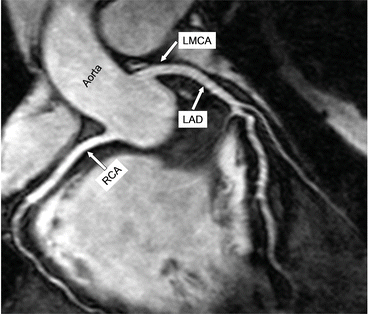
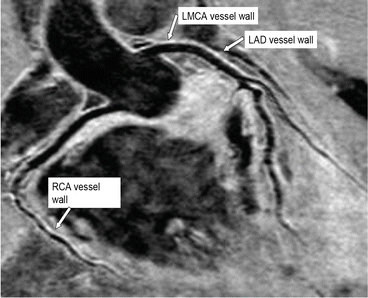

Fig. 6
In this healthy subject the proximal origin and course of the right coronary artery, left main coronary artery, and left anterior descending coronary artery are well visualized and seen to be free of any significant disease [6]. Please note that the vessel wall is imaged using the same CMR sequence (see Fig. 7). RCA right coronary artery, LMCA left main coronary artery, LAD left anterior descending coronary artery

Fig. 7




CMR imaging from the same healthy subject as shown in Fig. 6, with emphasis on imaging of the coronary wall, confirming no significant atherosclerotic process in the wall of the vessel [6]. The same CMR sequence can be used to visualize both the coronary lumen (Fig. 6) and coronary wall (Fig. 7)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree