Magnetic resonance enterography (MRE) is an established test in patients with Crohn because of its ability to display transmural enteric inflammation and treatment response throughout the gastrointestinal tract without the very low potential risk of ionizing radiation. This article discusses how and when to diagnose Crohn disease with MRE (including discussion of the main differential diagnosis), how to describe the burden of enteric inflammation and its complications, and how to accurately classify disease based on interdisciplinary consensus. In addition, brief overviews of expected future MRE developments and alternative imaging modalities are also discussed.
Key points
- •
Magnetic resonance enterography (MRE) should be performed at the time of Crohn’s diagnosis to detect small bowel inflammation, strictures, and penetrating complications that are not detected by ileocolonoscopy and serum or fecal markers.
- •
Active inflammatory small bowel Crohn’s disease should be diagnosed when bowel wall thickening and segmental hyperenhancement coexist in a known patient with Crohn’s, are present with typical penetrating or stricturing complications, or are present asymmetrically in the bowel wall along the mesenteric border.
- •
When small bowel Crohn’s disease is present, radiologists should describe its location, length, and severity, because these important parameters influence treatment decisions.
- •
In the presence of a stricture (defined as an area of diseased luminal narrowing associated with unequivocal upstream dilatation >3 cm), presence of active inflammation, length, degree of upstream dilatation, presence of associated penetrating complications, and enteric anastomoses should be described.
- •
Expected areas of advancement including MRE involve development of (1) biomarkers capable of predicting drug response and outcomes, (2) pulse sequences able to measure wall fibrosis, (3) classifiers of disease using MRE pulse sequences without the use of intravenous contrast.
Introduction
Crohn’s disease (CD) is a chronic inflammatory condition involving predominantly the gastrointestinal (GI) tract, with increasing incidence worldwide. Recently developed targeted biologic agents result in the ability to improve long-term outcomes and reduce patient morbidity. Consequently, there is a growing need to monitor drug and antibody levels and predict (and ensure) treatment response. Deep and long-lasting remission from enteric inflammation is the goal of therapy, and therapeutic monitoring can avoid underuse and detect nonresponse. Besides imaging, the main tools gastroenterologists have at hand are patient symptoms, therapeutic drug monitoring, endoscopic/histologic assessment, and serum/fecal markers. However, many symptomatic indices are prone to interobserver variability, incorporate subjective terms such as well-being or abdominal pain, and do not reflect objective markers of inflammation burden such as endoscopy and histology. Consequently, additional objective measures of active disease, response to treatment, and complications are needed.
Magnetic resonance enterography (MRE) is now an established test in patients with Crohn’s because of its ability to show transmural enteric inflammation and treatment response throughout the GI tract without the very low potential risk of ionizing radiation. This article provides a road map for how and when to diagnose CD with MRE (including discussion of the main differential diagnoses), how to describe the burden of enteric inflammation and its complications, and how to accurately classify disease based on interdisciplinary consensus. In addition, brief overviews of expected future MRE developments and alternative imaging modalities are also discussed.
Adaptation to standard imaging protocols
Optimal small bowel magnetic resonance (MR) imaging protocols in general are discussed comprehensively elsewhere (see Darren Boone and Stuart A. Taylor’s article, “ MR of the Small Bowel: How to Do It ,” in this issue). Recommendations on MRE protocols have been published by the Society of Abdominal Radiology (SAR) and by the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) in consensus with the European Crohn’s and Colitis Organisation (ECCO) ( Table 1 ). Specific considerations based on patient age and on particular clinical questions (presence of perianal disease or existence of significant fibrosis in Crohn’s lesions) are discussed later.
| SAR | ECCO-ESGAR | |
|---|---|---|
| Patient preparation | • Fasting for 2–4 h | • Fasting for 4–6 h |
| Equipment | • Not mentioned |
|
| Anatomic coverage |
| • Not mentioned |
| Bowel paralysis |
|
|
| Enteric contrast |
|
|
| Mandatory sequences |
|
|
| Optional sequences |
|
|
a Routine MRE protocols do not thoroughly delineate perianal fistulae and their relationship to the sphincteric complexes, but can detect/exclude most penetrating complications.
Adaptations for the pediatric population
Patient cooperation is essential to achieving diagnostic quality MRE in children and adolescents. Education preceding MRE, and active encouragement during the examination, can optimize compliance by helping ensure proper fasting for 4 to 6 hours, drinking the required volume of enteric contrast material, staying still, and good breath holding.
Weight-based protocols are used in pediatric patients to determine the volumes of enteric and intravenous (IV) gadolinium-based contrast agents (GBCAs) and dose of antispasmodic agent administered. Enteric contrast is administered as 3 boluses over 45 to 60 minutes. Providing patients with a choice of enteric contrast agents improves compliance. These hyperosmolar contrast agents provide comparable but improved distension compared with water and are often more palatable when chilled.
The antispasmodic agents glucagon and hyoscine-butyl-bromide (Buscopan) are both used in pediatric patients; Buscopan is off label in children but may be slightly better tolerated. These agents are administered as 1 or 2 doses before GBCA.
Imaging acquisition following intravenous GBCA injections can be dynamic postcontrast, enteric phase (45 seconds), portal venous phase (70 seconds), and/or delayed up to 7 minutes, similar to adults.
Imaging sequences used in pediatric MRE are the same as those detailed for adult MRE protocols, with acquisition parameters adapted to patient size. General anesthesia may be needed for MRE in younger patients (eg, <6 years old) and in those with impaired cognition or developmental delay. Modifications for general anesthesia include intubation; enteric contrast given via orogastric or transpyloric tube in multiple doses before image acquisition; and avoidance of antispasmodic agents, which can potentiate anesthesia-related ileus.
Combined magnetic resonance enterography and perianal protocols
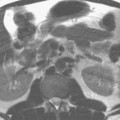
Standard MRE protocols are useful to screen the perianal region (the perianal region should be imaged as part of standard MRE examinations), but may not completely exclude or classify perianal fistulas without dedicated pelvic/perianal imaging. AlSabban and colleagues found that MRE had a high sensitivity of 82% and very high specificity of 100% for detection of any perianal disease, but did not identify some fistulae and small-volume abscesses, so MRE also is insufficient for fistula classification ( Fig. 1 ). There is an additional time penalty by combining pelvic MR imaging with MRE that can result in increased motion artifacts or be complicated by incontinence; however, adding a limited number of small-field-of-view, multiplanar T2-weighted images to routine MRE may improve fistula detection.

Techniques for identification of bowel wall fibrosis
The presence of strictures is commonly an indication for endoscopic or surgical intervention, and the ability to detect and measure intestinal fibrosis in strictures would be helpful for directing the medical and surgical management; however, fibrosis is challenging to identify using conventional MR imaging techniques.
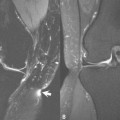
Barkmeier and colleagues concluded that the degree of small bowel dilatation proximal to an area of luminal narrowing of more than 3 cm was highly associated with confluent transmural fibrosis on histopathologic assessment following stricture resection ( Fig. 2 ). Rimola and colleagues evaluated change in bowel wall enhancement over time and found that progressive stricture enhancement between 70 seconds and 7 minutes and homogeneous enhancement on delayed images correlate with histologic fibrosis. Animal as well as human studies have also suggested that magnetization transfer (MT) MR imaging can be used to detect and estimate the amount of bowel wall fibrosis. Increased macromolecule concentration, such as collagen, causes a tissue’s MT ratio (MTR) to increase, allowing MTR to be used as a surrogate for bowel wall fibrosis. Li and colleagues have extended prior work and shown that MTRs normalized to skeletal muscle are significantly higher in fibrotic bowel segments in patients with CD. Ultimately, additional research is needed to establish which of these methods is best for detecting and measuring bowel wall fibrosis.

Imaging findings and pathology
Crohn’s Disease and How It Affects the Bowel
CD is one of the major subtypes of inflammatory bowel disease (IBD) and is characterized by active, chronic, relapsing inflammation of the GI tract, of multifactorial cause including different genotypes, with several monogenetic mutations now recognized in children less than 6 years old with very-early-onset IBD (VEO-IBD). Although ulcerative colitis (UC) is limited to the colon, CD can affect anywhere from the mouth to the anus, most often small and large bowel (usually discontinuous), involves the adjacent mesentery, with inflammation often more pronounced along the mesenteric border. At a microscopic level, active inflammation of the GI tract in CD is seen as a patchy transmural process, with the presence of nonnecrotizing, noncryptolytic epithelioid granulomas in mucosal and/or submucosal lymphoid tissue, pivotal to distinguishing CD from UC.
Bowel wall inflammation variably progresses to stricturing and/or penetrating disease, the hallmarks of CD (typically a transmural process), differing in number of involved segments, location, extent, and severity over time and between patients.
The Montreal and Paris classification systems are used to define the spectrum of different CD phenotypes seen in adults and children, respectively, but do not adequately summarize the coexistence of inflammation, inflammation severity, and stricturing/penetrating complications seen on MRE.
Crohn’s Disease Presentation, Progression and Stricture Formation
Classic clinical findings of CD are abdominal pain, diarrhea, and weight loss, but few patients have all three at presentation, often leading to a delay in diagnosis. Unexplained anemia presenting as fatigue, fever, and linear growth impairment warrant particular attention in children, as does secondary amenorrhea. Around 20% to 26% of adults initially present with a penetrating or obstructing complication compared with approximately 10% in children, who may also present with cutaneous CD. Disease progression varies with time, therapy, and location, with ileal disease more often leading to stricture (from smooth muscle hypertrophy and collagen deposition) and pseudosacculation. Obstruction, with worsening pain and vomiting, can occur with bowel wall thickening and edema from active bowel inflammation or mixed inflammatory and fibrotic strictures (see Fig. 2 ).
Spectrum of Penetrating Disease
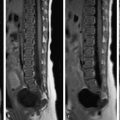
Penetrating disease occurs with progression of CD, more often in patients having frequent flares, and manifests as sinus tracts, fistulas, inflammatory masses, and abscesses, being accurately detected with contrast-enhanced MRE ( Fig. 3 ). Recent data have shown that internal penetrating disease is associated with luminal narrowing/bowel stricturing and sites of active bowel inflammation.

Associations with Perianal Disease
VEO-IBD is very rare (3%) but colonic and especially perianal disease predominates in this setting. Although perianal disease is present in a substantial minority of children and adults at diagnosis, it may be the only manifestation of CD in 5%. The incidence of perianal disease increases the closer colonic inflammation is located to the anus, and perianal fistulas do not heal when proctitis is present. In children, a distinct phenotype is characterized by perianal disease in conjunction with an overall worse burden of rectal and jejunal inflammation.
Other Perienteric/Extraintestinal Complications
Perienteric features of CD can be a sign or a sequela of active inflammation, and include regional fibrofatty proliferation, also known as creeping fat; engorgement of the vasa recta, or “comb” sign; mesenteric enhancement and edema; and, rarely, mesenteric venous thrombosis if acute, and mesenteric venous occlusions with or without varices if chronic. Extraintestinal manifestations of CD include gallstones, renal stones, pancreatitis (eg, autoimmune or caused by immunosuppression with azathioprine) and spondyloarthritis, and can be detected with MRE. Primary sclerosing cholangitis occurs most commonly in conjunction with IBD, and is of prognostic importance because of poor survival outcomes; therefore suspicion of intrahepatic or extrahepatic bile duct dilatation or beading on MRE should prompt dedicated imaging with magnetic resonance cholangiopancreatography.
Diagnostic criteria
MR imaging features correlating best with active inflammatory CD of the bowel include segmental mural hyperenhancement and mural thickening, especially if asymmetric (affecting predominantly the mesenteric border), T2-weighted signal hyperintensity (reflecting intramural edema), restricted diffusion on diffusion-weighted imaging (DWI), and luminal ulcers (breaks in the inner wall of the small bowel with associated intramural extension of air or enteric contrast ( Figs. 4 and 5 ). These imaging findings correlate with endoscopic and histologic evidence of active inflammation in adults and children, and are reflected in MR imaging indices used to score inflammatory disease severity such as the MR index of activity and London scores. Because imaging findings are frequently nonspecific, intersociety recommendations state that active inflammatory Crohn’s small bowel disease should be diagnosed when bowel wall thickening and segmental hyperenhancement coexist in a known patient with Crohn’s, are present with typical penetrating or stricturing complications, or are present asymmetrically in the bowel wall predominantly along the mesenteric border (see Figs. 5 and 6 , Table 2 ). Restricted diffusion is not part of the diagnostic criteria for CD but reflects inflammation severity (see Fig. 4 ). In the jejunum, asymmetric inflammation distorts or effaces the normal fold pattern (see Figs. 6 and 7 ). Nonspecific imaging findings can be correlated with clinical symptoms and endoscopic findings and followed over time to determine their import.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree