Small bowel magnetic resonance (MR) imaging has been clinically implemented for many years, albeit with variation in study technique. Considerable research has been performed during this time regarding optimum patient preparation, choice of enteric contrast medium, and MR imaging sequence protocol but findings have not been universally implemented. However, evidence-based consensus statements have recently been published from the United States and Europe. This article summarizes key findings from this guidance and presents practice examples from the authors’ own institution.
Key points
- •
Small bowel magnetic resonance (MR) imaging has been used in routine practice for nearly 2 decades.
- •
Implemented protocols still vary in patient preparation, enteric contrast, and MR imaging acquisition sequences, resulting in heterogeneous diagnostic quality.
- •
Consensus statements from the United States and Europe have recently been published to inform evidence-based small bowel MR imaging technique.
Introduction
Dedicated small bowel evaluation with magnetic resonance (MR) imaging has been in routine use for nearly 2 decades. Once MR imaging technology advanced sufficiently to enable rapid acquisition of abdominopelvic sequences minimizing motion artifacts from peristalsis and respiration, it naturally followed that dedicated protocols to interrogate the small bowel would be performed following enteric distention with contrast medium, either administered orally (enterography) or via a nasoenteric tube (enteroclysis). As the technique evolved, many units adapted their existing abdominal imaging protocols for small bowel MR imaging, using differing bowel preparations and acquisition parameters. Consequently, heterogeneity developed in terms of institutional protocols with resulting variability in the diagnostic quality of the studies obtained. However, well-researched consensus statements have recently emerged from North America and Europe providing evidence-based guidance on the optimal small bowel MR imaging technique ( Table 1 ). Although a detailed description of respective methodologies is beyond the scope of this article, both groups assembled panels of expert contributors and performed systematic reviews of the available literature to develop a comprehensive set of consensus recommendations. Therefore, this article presents the available research relating to the optimum small bowel MR imaging technique, summarizes the relevant US and European consensus guidance, and shares experience from the authors’ daily practice.
| Patient Preparation and Basic Technique | |
| Patient preparation: general | Routine medications should not be stopped (V) |
| Patients should not eat any solid food for 4–6 h (V) | |
| Patients should not drink fluids for 4–6 h except nonsparkling water (V) | |
| Technique: enterography | There is no single preferred contrast agent for MR enterography |
| Recommended agents include mannitol, PEG, sorbitol, and lactulose (III) | |
| The optimal volume of oral contrast is 1000–1500 mL (III) | |
| Ingestion time should be 45–60 min (V) except when previous small bowel resection | |
| If there is a stoma, it should be plugged before oral contrast ingestion (V) | |
| Laxative bowel preparation is not recommended (V) | |
| Water enema or prolonged oral contrast preparation is suggested for dedicated colonic evaluation | |
| The volume of a water for rectal enema should be based on patient tolerance | |
| Technique: MR enteroclysis | There is no single preferred contrast agent for MR enteroclysis. Recommended agents as above |
| Fluoroscopic guidance for NJ tube insertion before enteroclysis is mandatory (V) | |
| The NJ tube should be 8–10 F (V) | |
| Enteric contrast infusion should be via an automated pump (V) | |
| The rate of contrast infusion before MR enteroclysis should be between 80 and 120 mL/min (V) | |
| MR imaging fluoroscopic monitoring of small bowel filling during MR enteroclysis is mandatory (V) | |
| Enteric contrast progression should be monitored on the MR imaging table during MR enteroclysis (V) | |
| The optimal volume of enteric contrast should be based on real-time monitoring (V) | |
| MR Enterography/Enteroclysis Technical Considerations and Sequence Selection | |
| Hardware | Both 1.5 and 3 T are adequate field strengths (II) |
| The use of phased-array coils is mandatory (V) | |
| Spasmolytic agents | Spasmolytic agents are recommended (II) |
| Timing of administration should account for motion-sensitive sequences (V) | |
| The recommended first-line spasmolytic agent in Europe is 20 mg IV hyoscine butylbromide (V) | |
| The recommended second-line agent is 1 mg IV glucagon (V) (first line in United States) | |
| Positioning | Patients can be scanned prone or supine (III) |
| Recommended sequences | Axial and coronal T2 FSE without FS |
| Axial and coronal SSFPGE without FS | |
| Axial or coronal T2 FSE with FS | |
| Before and after IV contrast-enhanced 3D T1-weighted gradient-echo sequence with FS | |
| IV contrast enhancement | In known or suspected IBD, sequences should be in the enteric (45 s) or portal venous phase (70 s) |
| For suspected chronic GI bleeding, contrast-enhanced sequences should be in the arterial (30 s), enteric (45 s) or portal venous phase (70 s) phase | |
| IV contrast media should be pump injected with an infusion rate of 2 mL/s and a dose of 0.1–0.2 mmol/kg (V) | |
| Optional sequences | Cine motility and DWI are suggested but not mandatory (V) |
| DWI should use a free-breathing technique (IV) | |
| DWI sequences should include b values ranging from 0 to 900 (IV) | |
| Maximal slice thickness for DWI should be 5 mm (V) | |
| Coronal diffusion-weighted sequences are not recommended (V) | |
| Dynamic contrast-enhanced sequences are suggested but not mandatory (V) | |
| Magnetization transfer sequences are not recommended (V) | |
| Parameters | Maximal slice thickness for FSE T2W and SSFP GE sequences should be 5 mm (V) |
| FSE T2W sequences may be performed in either 2D or 3D, although 2D is preferred (V) | |
| Maximal slice thickness for axial and coronal T1W sequences, should be 3 mm (V) | |
| T1W sequences should be performed in 3D (V) | |
| Scan coverage and duration | Coverage should include at least the small bowel, colon, and perineum (V) |
| Total acquisition time should be 30 min or less (V) | |
General patient preparation and basic magnetic resonance imaging technique
The aim of pretest preparation is to achieve maximal small bowel distension with enteric contrast medium while minimizing the artifacts that result from peristalsis or excessive luminal gas through use of spasmolysis and fasting.
Enteric Contrast Administration
Pretest preparation
Although many clinicians consider that fasting for 4 to 6 hours improves tolerance of oral contrast medium, there is no strong supportive evidence. Fasting for 2 to 4 hours may be sufficient. Nonetheless, it is universally accepted that patients should continue their regular oral medication and avoid ingestion of carbonated drinks because of the risk of producing intraluminal gas artifacts. Clear fluids are generally thought to be acceptable; the authors find that a 4-hour solid food fast improves oral contrast ingestion and image quality while remaining well tolerated.
Enteric contrast medium
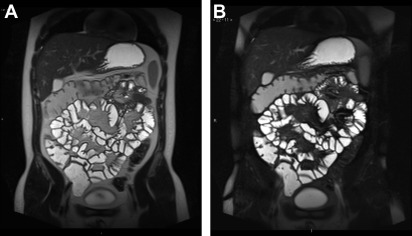
There is good evidence that the accuracy of MR imaging is improved by administration of oral contrast and this is currently considered the standard of care recommended by both US and European consensus guidance ( Fig. 1 ). Collapsed bowel can both hide and mimic small bowel disease.

Choice of contrast agent
Ideal enteric contrast media should maximize luminal distension along the entire small bowel for the duration of the study. Water is generally insufficient. Hyperosmolar agents reduce gut absorption and viscous fluids tend to promote distension. Although so-called dark lumen imaging using superparamagnetic iron oxide nanoparticles ( Fig. 2 ) is favored by a few centers, use of media that produce characteristic luminal T2 hyperintensity to achieve maximum contrast against the bowel wall ( Fig. 3 ) is now almost universal. Such agents are hypointense to the bowel wall on T1-weighted sequences such that bowel wall enhancement is readily appreciated ( Fig. 4 ). Consequently, several oral contrast agents have been tested and their resulting properties described in the literature, but no strong evidence from patient cohorts supports one particular agent more than another. Nevertheless, a consistent finding is that distension is improved by hyperosmolar agents, particularly when ingested between 45 and 60 minutes before the examination.


Commonly used T2-hyperintense contrast media include polyethylene glycol (PEG) and mannitol solution in varying concentrations, often prepared locally with or without bulking agents such as locust bean or xanthan gum. In the United States, 0.1% low-density barium sulfate suspension is also commonly administered. Available superparamagnetic iron oxide contrast agents are proprietary formulations subject to local regulatory approval and much less frequently used. The European consensus statement states that, given the lack of evidence suggesting superiority of one preparation compared with another, the decision is left to clinician preference. In our practice, the authors find a simple 2.5% mannitol solution offers reproducible signal characteristics, luminal transit, and distension without compromising patient acceptability, cost, or safety. In addition, we have seen that refrigeration and flavoring (eg, with blackcurrant cordial) enhances compliance, particularly in children. It should be remembered that hyperosmolar agents often cause abdominal bloating and diarrhea, which are often identified by patients as the worst part of the examination. Patients should therefore be appropriately counseled about such side effects and given advice on symptom management, postexamination travel, and so forth.
Contrast volume and timing
Limited evidence relates to optimal enteric contrast volume. For example, a study of 10 healthy volunteers showed that volumes of less than 1000 mL resulted in inferior distension, whereas others have shown that reasonable-quality examinations can be obtained with as little as 450 mL of oral contrast media. Consequently, neither US nor European panels reached consensus on the minimum acceptable enteric oral contrast volume; in clinical practice, this is usually judged on a case-by-case basis. Similarly, scant evidence exists for the optimal duration of enteric contrast ingestion and whether to split into divided doses or drink continuously. The authors aim to administer 1600 mL of 2% mannitol solution over 1 hour for routine small bowel studies and 1000 mL over 45 minutes if there has been extensive prior small bowel resection. It is imperative that the need for consumption of oral contrast is explained to patients and advice given on the methods of ingestion over the preexamination time period. In our experience, such counseling improves the quality of examinations. If an ileostomy is present, plugging is recommended by European guidance to improve enteric distension. However, there is no direct evidence to support this approach and, for pragmatic reasons, the authors do not routinely do this.
Use of rectal enema
Although there is some evidence that use of rectal contrast administration may assist in demonstration of the ileocecal junction, this is not recommended. Specific colonic evaluation as part of small bowel MR imaging protocols is discussed in further detail later.
Enteroclysis
Enteroclysis is designed to achieve optimal luminal distension via direct administration of enteric contrast into the jejunum via a nasojejunal tube. Some studies have shown improved small bowel distension compared with routine oral contrast administration, but a meaningful impact on clinical decision making has not been shown. Furthermore, because enteroclysis requires placement of a nasoenteric feeding tube, usually under fluoroscopic control, it is invasive and hence potential improvement in technical quality must be weighed against impact on compliance and radiation exposure.
The optimal volume of enteric contrast for MR enteroclysis should be based on real-time monitoring; delivery via an automated pump system is preferred but not mandatory. In all other respects, international guidance essentially mirrors that of MR enterography for patient preparation, positioning, and acquisition protocol. Overall, although acknowledging this technique can be valuable (eg, to show adhesions or luminal filling defects such as polyps), enteroclysis is not considered necessary for effective small bowel MR imaging in inflammatory bowel disease by either US or European consensus guidance.
Technical considerations and sequence selection
Patient Positioning
Although some data suggest superior luminal distension can be achieved when prone, and that prone positioning may decrease coronal sequence acquisition time by compressing the abdomen, there is no hard evidence that this improves diagnostic accuracy. Moreover, some patients find this position difficult, particularly those with a stoma, hence supine positioning is also considered acceptable. In our practice, the authors attempt prone imaging wherever possible because we observer fewer respiratory motion artifacts and improved acceptability for those who find MR imaging claustrophobic.
Spasmolysis
Although some studies have suggested that satisfactory small bowel MR imaging can be achieved without the use of a spasmolytic, many show significantly superior distension with the use of these agents, particularly proximally. Furthermore, reduction in peristalsis-related motion artifact improves study quality. International guidance therefore recommends spasmolysis before MR enterography. The evidence suggests that both glucagon and hyoscine butylbromide are acceptable, albeit with differing onset and duration of effect. Both are most effective when given intravenously.
Although data from healthy volunteers suggests that glucagon is superior to hyoscine butylbromide for achieving complete aperistalsis, there is currently no evidence that this improves diagnostic accuracy. Therefore, based on expert opinion, cost, and availability, in Europe, 20 mg of hyoscine butylbromide is recommended as the first-line spasmolytic, with 1 mg of glucagon as second line. Either a single or a split dose is acceptable. In the United States, where regulatory approval differs, intravenous (IV) glucagon is considered first line. Because of the short half-life, spasmolytics should be administered directly before motion-sensitive sequences, such as T2-weighted imaging and three-dimensional (3D) T1-weighted postcontrast series (postcontrast-enhanced images are particularly sensitive to peristaltic motion artifact). Intramuscular administration can also be considered if necessary; it is longer lasting but less predictable. Both EU and US guidance mandate evaluation for contraindications or drug interactions before antispasmodic and contrast administration. The authors’ current MRE protocol is summarized in Table 2 .
Magnetic Resonance Imaging Acquisition
Hardware
There is currently no EU or US consensus regarding optimal field strength for small bowel MR imaging; available evidence confirms that high-quality acquisitions are possible at both 3 T and 1.5 T. The use of phased-array coils is recommended regardless of field strength. The authors routinely perform enterography at both field strengths, using an extralarge torso coil, with slight parameter modifications as detailed in Tables 3 and 4 .
| Patient Preparation | Continue routine medications |
| No solid food for 4 h; nonsparkling water only for 4 h | |
| Patient arrives 1 h before scan | |
| Enteric contrast; 1600 mL of 2% mannitol over 40 min (or 2500 mL in 2 divided doses over 3 h in specific cases in which dedicated colonic imaging is indicated; see main text) | |
| Patient consent and suitability for IV spasmolytic and IV gadolinium contrast enhancement confirmed (if indicated) | |
| Patients positioned prone with arms above head if possible; feet first if claustrophobic | |
| Scanning Technique | 1.5-T or 3-T platform with large torso phased-array coils |
| Scan coverage is from diaphragm to perineum; if detailed perineal imaging is required, additional fistula protocol | |
| Total acquisition time is just <30 min | |
| Sequences | Sequences acquired as per Table 3 (1.5 T, Siemens Avanto) and Table 4 (3T Philips Achieva) |
| Additional sagittal cine sequences if adhesions are suspected | |
| IV gadolinium administration only in known/suspected penetrating disease. If administered, pump injection of 2 mL/s infusion rate, dose of 0.1 mmol/kg and images acquired at 70 s |
| Step | Sequence | Plane | 2D/3D | ST (mm) | TR | TE | FS |
|---|---|---|---|---|---|---|---|
| 1 | Localizer | ||||||
| 2 | T2 TRUFI coronal BH 4 mm | Cor | 2D | 4 | 3.45 | 1.46 | No |
| 3 | T2 TRUFI transverse BH 4 mm | Ax | 2D | 4 | 3.73 | 1.87 | No |
| 4 | T2 TRUFI coronal BH 20 measures (motility study) | Cor | 2D | 10 | 3.57 | 1.79 | No |
| 5 | Hyoscine Butylbromide 10 mg | ||||||
| 6 | T2 HASTE coronal BH 4 mm | Cor | 2D | 4 | 600 | 87 | No |
| 7 | T2 HASTE coronal BH 4 mm FS | Cor | 2D | 4 | 500 | 87 | Yes |
| 8 | T2 HASTE transverse BH 4 mm | Ax | 2D | 4 | 500 | 87 | No |
| 9 | T2 HASTE transverse BH 4 mm FS | Ax | 2D | 4 | 500 | 87 | Yes |
| 10 | DWI ep2d diff 3av (b values 0, 600; 3 averages) | Ax | 2D | 5 | 2500 | 85 | Yes |
| 11 | Hyoscine Butylbromide 10 mg if IV Contrast Indicated | ||||||
| 12 | T1 VIBE coronal BH FS precontrast | Ax | 3D | 3.5 | 3.24 | 1.1 | Yes |
| 13 | IV Gd Contrast Administration | ||||||
| 14 | T1 VIBE coronal FS BH postcontrast | Cor | 3D | 3.5 | 3.24 | 1.1 | Yes |
| 15 | T1 VIBE FS transverse BH postcontrast | Ax | 3D | 3 | 4.89 | 2.39 | Yes |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree