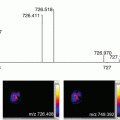
Fig. 1
Sample stocking procedure for ITO glass slides with mounted tissue
3.1.2 Tissue Preparation for MALDI-MSI
- 1.
Following removal from the freezer, the ITO glass slides with mounted sections are dried via desiccation for 30–60 min (based upon the thickness and dimensions of the section). Vacuum dehydration removes excessive moisture from the surface of the tissue that may occur during the thaw-mounting process, thus reducing the possibility of analyte delocalization.
- 2.
The excess OCT compound is removed from the edges of the tissue by using a swab that has been briefly dipped in a 70% EtOH solution. Be careful when approaching the edges of the tissue, if too much force is used the edges of the tissue may be broken and detached from the ITO glass slide (see Notes 1 and 2 of Sect. 4).
- 3.
The tissue sections are then placed sequentially into petri dishes containing increased concentrations of Ethanol (A, B, C) and gently agitated for 30 s during each wash. Excess Ethanol is then quickly removed from the slide by wiping gently with tissue paper.
- 4.
Teaching points are positioned on the corner of the slide/target prior to scanning. Scanning of the tissue is performed prior to matrix deposition in order to obtain a clear image of the tissue borders. The resolution of the scanned image should be selected based upon the spatial resolution of the planned MALDI-MSI analysis (2400 dpi is usually sufficient for 30 μm spatial resolution MALDI-MSI acquisitions).
- 5.
The SA matrix solution is deposited onto the tissue using the iMatrix Spray automated spraying device. An example “dry spray” method involves the following parameters; Spray height: 60 mm, Speed: 180 mm/s, Line distance: 1 mm, Density: 2 μL/cm2, Break: 60 s and Number of cycles: 5 [11] (see Note 3 of Sect. 4).
- 6.
When conductive ITO glass slides are used, matrix is removed from the edges of the slide using Methanol and tissue paper in order to avoid uneven conductivity of the slide. Matrix is also removed from a small area near to the tissue in order to add protein calibrants.
- 7.
A solution containing a 1:1 ratio between SA and the protein calibrant is prepared. This protein calibrant is then spotted onto a clean area of the ITO slide/target.
3.1.3 MALDI-MSI Analysis
- 1.
The previously positioned teaching points are used for teaching the instrument and to set the X and Y-axes. The measurement region is then set using FlexImaging software and information related to the geometry is sent to the instrument using FlexControl. Other parameters, such as the rastering, are configured in FlexImaging and selected based upon the dimension of the histological features to be analyzed and the crystal dimensions. Considering the dimensions of glomeruli, a raster of ≤50 μm is recommended. For each raster position, a mass spectrum is acquired by accumulating approximately 500 shots. The number of shots should be carefully selected by the user depending upon the amount of matrix deposited and the fragility of the tissue.
- 2.
Following calibration using the protein standard, the selected section is analyzed using a Bruker UltrafleXtreme mass spectrometer equipped with a Smartbeam laser (Nd:YAG, 355 nm wavelength, 1–2 kHz frequency) and operated in positive linear mode in the mass range of 3–20 kDa.
3.1.4 Histological Evaluation
- 1.
Following MALDI-MSI analysis (Fig. 2), the matrix can be removed from the tissue by washing sequentially in increasing concentrations of Ethanol (A, B, D) and gently agitated for 30 s during each wash . Excess Ethanol is then quickly removed from the slide by wiping gently with tissue paper.

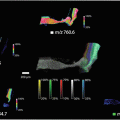
Fig. 2
(Left) Example renal biopsy that has been stained using Masson’s trichrome, with the pathological glomeruli (green circle) and tubules (green rectangle) annotated by the nephropathologist. (Right) MALDI-MSI representation from an annotated pathological region (dashed black rectangle)
- 2.
The tissue is then colored with the appropriate histochemical stain(s) (see Note 4 of Sect. 4) in order to highlight the particular morphological features that are desired in the study (see Table 1).
Table 1
Routine immunohistochemical stains used in the evaluation of renal biopsies
Immunohistochemical stain
Diagnostic feature(s) to be evaluated
Hematoxylin & Eosin (H & E)
General evaluation, cellular features, presence of inflammation, interstitial oedema, tubular and epithelial damage
Jones methanamine silver (JMS)
Glomerular basement membranes, tubulitis, miotic figures
Masson’s trichrome
Extracellular glomerular matrix, tubular basement membranes, fibrosis connective tissue, Bowman’ capsule
Periodic acid-Schiff (PAS)
General evaluation of glomeruli, glomerular basement membrane, Bowman’ capsule, mesangium, tubular basement membrane
- 3.
The stained tissue section is then digitally scanned for the direct overlap of histological and MALDI-MS images .
3.2 MALDI-MSI of Tryptic Peptides from Archived FFPE Tissue
3.2.1 Tissue Sectioning
- 1.
FFPE samples are fixed in formalin immediately following surgical excision. The time of fixation is related to the size and dimension of the tissue, but is most commonly between 24 and 48 h. Following formalin fixation, excess formalin is removed by washing the tissue with water. The tissue is then subjected to an automated fixation procedure involving ethanol dehydration, a xylene bath, and paraffin fixation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree