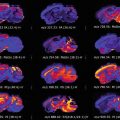
Benign thyroid nodules
Malignant thyroid nodules
Follicular adenoma (FA)
Conventional variant papillary thyroid carcinoma (cvPTC)
Hyperplastic (Hp)
Follicular variant papillary thyroid carcinoma (fvPTC)
Hashimoto’s thyroiditis (HT)
Uncertain malignant potential (UMP)
Mixed variant papillary thyroid carcinoma (mvPTC)
Fine needle aspiration (FNA) biopsies are the current gold-standard for the preoperative evaluation of thyroid nodules [6]. However, a significant number of them (15–30%) are unable to be affirmatively diagnosed and are given an “indeterminate for malignancy” final report, meaning that the malignant nature of the thyroid nodule remains unknown and the recommended therapeutic approach is total thyroidectomy. Furthermore, cytomorphological evaluation of biopsies taken post-surgery indicates that approximately 80% of nodules within this group of patients are in fact benign, and the performed thyroidectomy was consequently the incorrect therapeutic approach. Therefore, the identification of new diagnostic targets that can assist in the preoperative diagnosis of thyroid tumors, and ultimately reduce the number of unnecessary thyroid removals, is considered to be imperative.
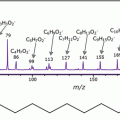
A number of proteomic approaches have been applied for the purpose of detecting new markers that can assist in the diagnostic characterization of thyroid nodules, employing both preoperative FNA biopsies and surgical specimens [7]. In particular, MALDI-MSI has the ability to provide spatially resolved information regarding protein expression in cytological specimens [6]. This enables the characterization of cell subpopulations based on their molecular profiles, even within regions that are indistinguishable at the microscopic level [8], and the feasibility of this approach to investigate ex vivo FNA specimens has already been highlighted [9, 10].
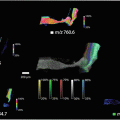
Mainini et al. performed a preliminary study on various types of nodules, including cvPTC, fvPTC, hyperplastic nodules, and Uncertain Malignant Potential (UMP) tumors, and attempted to differentiate these lesions by virtually microdissecting mass spectra from very discrete regions of cell populations associated with the malignant conditions and subsequently performing hierarchical cluster analysis (HCA) and principal component analysis (PCA) [9]. Complementary results were then obtained by Pagni et al. in their feasibility study, applying MALDI-MSI on a further set of nodules, including hyperplastic nodules, Hurtle cell-type FTA, MTC, and cvPTC [10]. The results showed that those nodules with similar biological behavior were represented by similar protein profiles , indicating that MALDI-MSI could potentially be employed to successfully classify these various thyroid lesions from a proteomic standpoint.
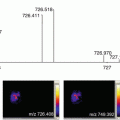
Of greater diagnostic relevance, Pagni et al. then attempted to distinguish benign lesions from malignant [11]. In this latest study, ex vivo fine needle aspirations from benign thyroid nodules and papillary thyroid carcinomas were analyzed by MALDI-MSI. Employing the protein profiles obtained from the aforementioned patients as a reference, MALDI-MSI was able to correctly assign, in blind, ten additional FNAB specimens to a malignant or benign class. This data suggested that an approach based on MALDI-MSI has the potential to play a significant role in the diagnosis of thyroid lesions and provide further insights into the development of tumorigenic activity.
Here, an overview on the sample preparation procedure for the MALDI-MSI analysis of ex vivo FNA biopsies is provided (Fig. 1). Although the workflow is similar to what would be required for the analysis of fresh-frozen tissue, there are specific points to consider given that the samples are cytological and can be prone to a number of interfering molecules and structures. Furthermore, given that the most biologically relevant information is present within only a small number of cells within the sample, the collaboration with the pathologist is of utmost importance. It is through the ability of MALDI-MSI to provide specific protein profiles of discrete cell populations, and the close collaboration with the pathologist, that the proteomic information from these small cellular aggregates can be exploited to provide an insight into the malignant nature of the thyroid nodule undergoing diagnosis.


Fig. 1
MALDI-MSI workflow for the analysis of cytological smears in the study of thyroid cancer
2 Materials
- 1.
25 G fine needles.
- 2.
Diff-Quick staining solution.
- 3.
Standard microscope glass slides.
- 4.
Indium Tin Oxide-coated glass slides (Bruker Daltonik GmbH, Bremen, Germany), or other MALDI targets (instrument compatible).
- 5.
Ethanol Solutions (A, B, C, D: 70, 90, 95, 100%).
- 6.
SA matrix solution: 10 mg/mL in 60/40 ACN:H2O w/0.2% TFA.
- 7.
Protein Calibration Standard: Angiotensin II, Angiotensin I, Substance P, Bombesin, ACTH clip 1–17, ACTH clip 18–39, Somatostatin 28 (Bruker Daltonik GmBH, Bremen, Germany).
- 8.
Methanol.
- 9.
Hematoxylin and Eosin.
- 10.
May-Grunwald Giemsa solution (for post-analysis staining of the cytological specimen).
2.1 Instrumentation
- 1.
Standard desktop digital scanner .
- 2.
Scanscope CS2 digital scanner (Aperio, USA).
- 3.
iMatrixSpray (for matrix deposition) (Tardo Gmbh, Subingen, Switzerland).
- 4.
MALDI Mass Spectrometer: UltrafleXtreme (Bruker Daltonik GmBH, Bremen, Germany).
2.2 Software
- 1.
FlexControl 3.4 (Bruker Daltonik GmBH, Bremen, Germany)—for setting the parameters for spectral acquisition.
- 2.
FlexImaging 3.0 (Bruker Daltonik GmBH, Bremen, Germany)—for image acquisition and visualization.
- 3.
3 Methods
3.1 Sample Procurement and Stocking
- 1.
Cytological samples are obtained from targeted nodules of thyroidectomy specimens, using fine needles (25 G), within 15 min after surgery. This procedure enables the procurement of cytological samples that contain a large number of thyroid cells, without artifacts and with minimal waste of thyroid tissue, to be obtained. Briefly, for each target nodule, different passes are performed in order to ensure that the nature of the nodule is accurately represented . These preliminary passes are stained with Diff-Quick in order to evaluate the adequacy of the cellular material . Once confirmed, the cellular material is drawn from the desired region of the nodule by the fine needle and deposited onto a conductive indium tin oxide (ITO) glass slide. Using a clean microscope glass slide, the droplet containing cellular material is then smeared along the surface of the ITO glass slide.
- 2.
Most commonly, specimens from both the nodule and the benign region of the thyroid, in a location opposite to the nodule, are taken. Given the variability associated with the sampling procedure, this enables more appropriate comparisons between malignant and benign cells.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree