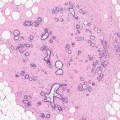
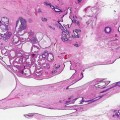
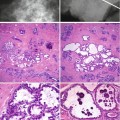
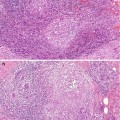
(1)
Princess Elizabeth Hospital Le Vauquiedor St. Martin’s Guernsey, Channel Islands, UK
(2)
Brighton and Sussex Medical School, Brighton, England UK
Abstract
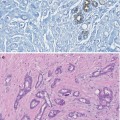
Gynaecomastia is a benign enlargement of the male breast due to glandular tissue proliferation manifesting as palpable firm mass. It is the most common reason for men attending the Breast Clinic. Gynaecomastia affects men of all ages. In two separate studies, gynaecomastia was detected in 36 % of healthy young men and 57 % of healthy older men on physical examination (Nuttall 1979) and more than 70 % of hospitalised elderly men (Niewoehner and Nuttal 1984). Autopsy studies reported the presence of gynaecomastia in 40–50 % of unselected cases (Andersen and Gram 1982).
Learning Points
Gynaecomastia occurs due to altered oestrogen–androgen imbalance.
Physiological gynaecomastia occurs during neonatal and pubertal periods.
Pathological gynaecomastia affects older men and has multifactorial causes.
Drugs and chronic illness promote the production/utilisation of oestrogens resulting in gynaecomastia.
Systemic and genetic factors promote the production of aromatase which converts androgens to oestrogens.
Gynaecomastia increases the risk of male breast cancer
15.1 Prevalence of Gynaecomastia
Gynaecomastia is a benign enlargement of the male breast due to glandular tissue proliferation manifesting as palpable firm mass. It is the most common reason for men attending the Breast Clinic. Gynaecomastia affects men of all ages. In two separate studies, gynaecomastia was detected in 36 % of healthy young men and 57 % of healthy older men on physical examination (Nuttall 1979) and more than 70 % of hospitalised elderly men (Niewoehner and Nuttal 1984). Autopsy studies reported the presence of gynaecomastia in 40–50 % of unselected cases (Andersen and Gram 1982).
Gynaecomastia has three peaks during a life span, thus: during neonatal period, during puberty and in older men. It is estimated that 60–90 % of infants have transient gynaecomastia due to transplacental transfer of maternal oestrogens. Neonatal gynaecomastia regresses completely by the end of the first year (Bembo and Carlson 2004). Gynaecomastia recurs in puberty when it affects 48–64 % of boys. It may affect boys as young as 10 years old and peaks between 13 and 14 years, followed by a decline in late teens (Bembo and Carlson 2004). The highest prevalence is men aged between 50 and 80 years (Nuttall 1979; Niewoehner and Nuttal 1984).
15.2 Causes of Gynecomastia
15.2.1 Altered Oestrogen–Androgen Balance
Gynaecomastia occurs due to an imbalance between oestrogen and androgens in favour of oestrogens or from increased sensitivity of the breast tissue to normal circulating oestrogen levels (Glass 1994). The imbalance is between the stimulating effect of oestrogen and the inhibitory effect of androgen (Ansstas et al. 2012). Oestrogens stimulate ductal epithelial proliferation, ductal elongation and branching, proliferation of periductal fibroblasts and increase in vascularity. The response to exposure of the male breast to oestrogens is similar to that of female breast (Braunstein 1993). Oestrogen production in men results from peripheral conversion of androgens (testosterone and androstenedione) to oestradiol and estrone through the action of aromatase mainly in the muscle, skin and adipose tissue (Ansstas et al. 2012). The second and small source of oestrogens is the testes. Altered oestrogen and androgen balance can be physiological, pathological or a genetic abnormality.
15.2.2 Physiological Gynecomastia
Neonatal gynaecomastia occurs in 60–90 % of infants due to transfer of maternal and placental oestrogen (Bembo and Carlson 2004). The second phase of physiologic gynaecomastia occurs at puberty and affects up to 60 % of boys (Bembo and Carlson 2004). At puberty there is a surge of luteinising hormone (LH) and follicle stimulating hormone (FSH) in conjunction with growth hormone and insulin-like growth factor-1 which stimulate testosterone production in Leydig cells. Oestrogen levels increase threefold, peaking earlier than testosterone concentrations and eventually increase to 30-fold (Niewoehner and Schorer 2008). It is uncertain whether gynaecomastia occurs due to delayed testosterone production, temporary increase in aromatase activity, varying sensitivity to oestrogen or a combination of these factors.
15.2.3 Pathological Gynaecomastia
Although gynaecomastia is idiopathic in 50 % of the patients (Einov-Bacher et al. 2004; Glass1994), pathologic gynaecomastia is caused by an increase in the production of oestrogen or decrease in the production of testosterone in conjunction with increased aromatisation (Ansstas et al. 2012). Several drugs also induce gynaecomastia. Decreased androgen action occurs with the following:
(i)
Decreased production
(ii)
Increased sex hormone binding globulin (SHBG), which binds testosterone and thus favours greater peripheral oestrogen action
(iii)
Androgen receptor antagonist
Increased oestrogen action occurs with the following:
(i)
Increased aromatisation of androgens to oestrogens
(ii)
Increased androgenic precursors (dehydroepiandrosterone (DHEA) and androstenedione
(iii)
Increased SHBG which binds testosterone and thus favouring greater peripheral oestrogen action
Primary hypogonadism due to mumps orchitis, trauma, cytotoxic chemotherapy or a congenital abnormality such as Klinefelter’s syndrome are associated with an oestrogen–androgen imbalance leading to gynaecomastia. The reduced levels of testosterone increase the levels of serum luteinising hormone (LH). The excess LH stimulates the aromatase enzyme in testicular Leydig cells to produce more oestrogen from testosterone. Furthermore, peripheral aromatisation of the adrenal androgens androstenedione to oestrogen remains unaffected (Ansstas et al. 2012; Bembo and Carlson 2004). Eighty percent of patients with Klinefelter’s syndrome develop gynaecomastia.
Secondary hypogonadism due to hypothalamic or pituitary abnormality is a rare cause of gynaecomastia. These patients have low LH production resulting in reduced testosterone and oestradiol in the testis, but the aromatisation of the adrenal cortex androgens continues (Ansstas et al. 2012; Bembo and Carlson 2004).
Several tumours, benign and malignant, are associated with gynaecomastia. Leydig cell tumour of testis, 90 % of which are benign, secretes oestradiol which suppresses LH leading to reduced serum testosterone. Excess oestradiol also stimulates SHBG which preferentially binds testosterone and thus reduces free testosterone paving the way for increased oestradiol activity (Bembo and Carlson 2004).
Human chorionic gonadotropin (HCG)-producing tumours are associated with gynaecomastia. HCG has similar action to LH and stimulates the Leydig cells to produce oestradiol. Several tumours secrete HCG, namely, testicular germ cell tumours, lung, liver and gastric cancer (Bembo and Carlson 2004). Several drugs induce gynaecomastia, the most well-known being diethylstilbestrol for treatment of advanced prostate cancer (Moore et al. 1945). Other drugs which induce gynaecomastia and their mode of action are listed in Table 15.1.
Table 15.1
Drugs associated with gynaecomastia
Low androgen levels: inhibition of testosterone synthesis |
Ketoconazole, metronidazole, gonadotropin releasing hormone agonists (chronic) and antagonists, spironolactone, chemotherapy (cytotoxic drugs) |
Low androgen levels: inhibition of testosterone action |
Androgen receptor blockers-bicalutamide, flutamide, nilutamide, spironolactone, eplerenone, cyproterone |
5 α reductase inhibitors – finasteride, dutasteride |
H2 blockers and proton pump inhibitors – cimetidine, ranitidine, proton pump inhibitors; marijuana |
High androgen levels resulting in high oestrogen levels |
Androgen administration- excessive testosterone replacement, anabolic steroids, androgen containing contraceptives; human chorionic gonadotropin |
High oestrogen levels or oestrogen action |
Oestrogen administration, occupational exposure to oestrogen, oestrogen containing creams or cosmetics, isoflavones |
Phytoestrogens – cosmetics, soy products, beer, tea tree oil, lavender oil |
Oestrogen action – diethylstilbestrol, clomiphene, phenytoin, digitalis |
Other or multifactorial |
Angiotensin-converting enzyme inhibitors, alcohol, amiloride, amiodarone, amphetamines, calcium channel blockers, ciclosporin, diazepam, growth hormone, highly active antiretroviral therapy, heroin, methyldopa, isoniazid, reserpine, risperidone, theophylline, tricyclic antidepressants (increase prolactin levels) |
Other conditions associated with gynaecomastia include chronic renal failure, cirrhosis of the liver, hyperthyroidism and malnutrition (Hershkovitz and Liberman 2002; Ansstas et al. 2012; Niewoeher and Schorer 2008). Men with end-stage renal disease may have reduced testosterone and elevated gonadotropin hormones resulting in breast development. Gynaecomastia in cirrhosis occurs due to reduced catabolism of androgens which are converted to oestrogens in the peripheral tissue. Hyperthyroidism increases aromatase activity and levels of SHBG. SHBG binds androgens more avidly, allowing higher free levels of oestrogens to act on the peripheral tissue such as the breast. Malnutrition and starvation reduce gonadotropin hormones and testosterone levels and on refeeding the production of oestradiol outpaces the rise in gonadotropin hormones and testosterone, resulting in gynaecomastia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree