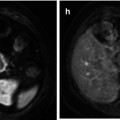
Fig. 59.1
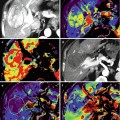
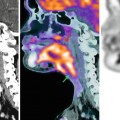
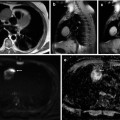
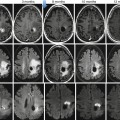
(a, b) A 50-year-old man presented with a black pigmented lesion in the left thigh. Clinical examination (a) revealed a black macule of 8–9 mm in diameter with irregular edges and variegate pigmentation, suggestive of superficial spreading melanoma. Dermoscopy (b): pigmented lesion with atypical network, streaks in periphery and blue-whitish veil, highly suggestive of melanoma. The histopathological analysis confirmed the diagnosis of superficial spreading melanoma 0.8 mm Breslow
The prognostic factors of CMM depend on the thickness of the lesion (Breslow index, Clark level), the presence of ulceration, the mitotic index, tumor location (located in the trunk and upper extremities have worse prognosis), the pattern of growth, histological type, age and sex, and involvement of regional lymph nodes. Among these clinical and pathological parameters, the histological and oncogenic status of the first node draining the primary tumor, also called “sentinel lymph node” (SLN), was found to be the most powerful prognostic factor in early-stage melanoma patients [4]. Early diagnosis of CMM, along with accurate staging of disease extent, is therefore essential for appropriate treatment decision making and, in turn, may give these patients the best chances of prolonged survival.
Nuclear medicine plays a key role in the correct nodal and distant staging of CMM. Sentinel lymph node biopsy (SLNB) and positron emission tomography/computed tomography (PET/CT) represent its main diagnostic tools.
59.2 Staging of CMM
In 2001, the American Joint Committee on Cancer (AJCC) released a revised version of the staging system for CMM (Tables 59.1 and 59.2), including the SLNB as part of staging. The AJCC staging system highlights the difference between clinical and pathologic staging. Therefore, clinical stage I and stage II disease refers to those patients with no evidence of nodal or distant metastases based on clinical and radiological evaluation. In contrast, pathologic staging is determined by information from both microstaging of the primary tumor and pathologic determination of lymph node (LN) status after either partial or complete lymphadenectomy [5].
Table 59.1
TNM staging
Primary tumor staging (T) | ||
Tx | Primary tumor is not identified | |
Tis | In situ melanoma | |
T1 | ≤ 1.00 mm | |
T1a | Without ulceration, without mitosis | |
T1b | With ulceration or mitotic rate ≥1/mm2 | |
T2 | 1.01–2.00 mm | |
T2a | Without ulceration | |
T2b | With ulceration | |
T3 | 2.01–4.00 mm | |
T3a | Without ulceration | |
T3b | With ulceration | |
T4 | >4.00 mm | |
T4a | Without ulceration | |
T4b | With ulceration | |
Lymph node status (N) | ||
N1 | One node involved | |
N1a | Micrometastases | |
N1b | Macrometastases | |
N2 | Two or three nodes affected | |
N2a | Micrometastases | |
N2b | Macrometastases | |
N2c | Intransit metastases/satellites without metastatic nodes | |
N3 | Four or more nodes or matted nodes or intransit metastases/satellites with metastatic nodes | |
Distant metastases (M) | ||
M1a | Distant skin, subcutaneous or nodal metastases with normal LHD levels | |
M1b | Lung metastases with normal LDH levels | |
M1c | All over visceral metastases or any distant metastases with elevated LDH levels | |
Table 59.2
2002 AJCC staging system for CM
Stage AJCC | Clinical staging | Pathologic staging | ||||
|---|---|---|---|---|---|---|
0 | Tis | N0 | M0 | pTis | N0 | M0 |
IA | T1a | N0 | M0 | pT1a | N0 | M0 |
IB | T1b | N0 | M0 | pT1b | N0 | M0 |
T2a | N0 | M0 | pT2a | N0 | M0 | |
IIA | T2b | N0 | M0 | pT2b | N0 | M0 |
T3a | N0 | M0 | pT3a | N0 | M0 | |
IIB | T3b | N0 | M0 | pT3b | N0 | M0 |
T4a | N0 | M0 | pT4a | N0 | M0 | |
IIC | T4b | N0 | M0 | pT4b | N0 | M0 |
IIIA | Any T | N1–3 | M0 | pT1–4a | N1a | M0 |
pT1–4a | N2a | M0 | ||||
pT1–4b | N1a | M0 | ||||
pT1–4b | N2a | M0 | ||||
IIIB | Any T | N1–3 | M0 | pT1–4a | N1b | M0 |
pT1–4a | N2b | M0 | ||||
pT1–4a/b | N2c | M0 | ||||
pT1–4b | N1b | M0 | ||||
IIIC | Any T | N1–3 | M0 | pT1–4b | N2b | M0 |
IV | Any T | Any N | M1 | Any T | Any N | M1 |
In 2009, the AJCC made a new revision in the staging of melanoma that included a third category, the rate of mitosis, which was added to the existing tumor thickness and ulceration. The mitotic rate is important to distinguish between T1a and T1b tumors. A T1b tumor is considered when the mitotic rate is ≥1/mm2. Also in this new classification, the presence of a single tumor cell in the SLNB is sufficient to classify as stage III [6].
59.3 Sentinel Lymph Node Biopsy
As previously discussed, the most important prognostic factor in patients with early-stage melanoma is the status of regional lymph nodes [7]. Only approximately 20 % of patients with an intermediate-thickness primary lesion are expected to have metastases in regional nodes. Almost 80 % of patients undergoing elective lymph node dissection (ELND) are at the risk for acute wound problems and chronic morbidities, such as lymphedema, nerve injury, and anesthetic complications, without actual survival benefit. SLNB was designed to identify occult nodal metastases and stage the regional nodal status, avoiding the morbidity of ELND in the 80 % of patients without regional nodal metastases. The initial experience with the SLNB was introduced in 1991 and published in 1992 by Morton et al. [8] as a new paradigm in the initial management of patients with early-stage cutaneous melanoma. The only international multicenter study of SLNB (MSLT-I) [9] was performed to evaluate the benefit of the technique in 1,269 patients with intermediate-thickness melanomas between 1994 and 2002. This study did not show an increase of overall survival in patients undergoing SLNB when compared to those that were not submitted to SLNB (87.1 and 86 %, respectively). However free rate of disease at 5 years was significantly higher in patients undergoing SLNB (78.3 vs. 73.1 %, P = 0.009). Moreover, in patients with positive nodes, the median survival was greater in those undergoing SLNB than in the other group (72.3 vs. 52.4 %, P = 0.004).
59.3.1 Sentinel Node Concept
Lymphatic mapping relies on the hypothesis that the dermal lymphatic drainage from cutaneous sites to the regional lymph node basin is an orderly process, and these lymphatic drainage patterns should mimic the metastatic spread of melanoma cells in the lymphatics. Therefore, the first lymph node(s) receiving lymphatic drainage (“the sentinel nodes”) is the most likely to contain metastatic disease [10]. A sentinel node (SN) is defined as a node upon which lymph vessel originating in the tumor drains directly [11]. Suggested other definitions of a SN in the literature include the node closest to the primary lesion, the first node visualized at lymphoscintigraphy, the hottest node, all radioactive nodes, all blue nodes, or all nodes with a count rate that is a certain factor higher than that of the background or compared to other nodes. Unfortunately, none of the definitions is perfect [11].
SLNB is a multidisciplinary procedure that requires close cooperation between nuclear medicine physicians, surgeons, and pathologists for accuracy lymphatic mapping, dissection and histopathologic analysis of the tumor draining nodes [12].
59.3.2 Indications
SLNB should be offered to patients with clinically localized disease and invasive melanoma, depending on different histopathologic characteristics of primary melanoma, including:
Thickness ≤1 mm: in patients with melanomas of 0.76–1 mm thick, SLNB indication differs according to different authors. Some authors systematically recommend SLNB, because the risk of nodal metastasis is approximately 5 %; others perform it in young patients with ulceration in the primary lesion or with a high mitotic rate [1]. In patients with a melanoma of ≤0.75 mm, usually SLNB is not recommended, as the risk of nodal metastases is around 1 %. Recently a group of authors recommended SLNB to all patients with CMM thicker than 0.5 mm [13].
Intermediate Thickness 1–4 mm: the risk of nodal metastasis increases from 8 to 30 %, and there is a consensus that SLNB should be performed in these patients [14].
Thickness >4 mm: these patients have the risk of distant metastases, and the risk of lymph node metastasis is around 40 %; the metastatic lymph nodes are still usually not clinically palpable. Therefore, these patients also benefit from SLNB [15].
SLNB is also indicated when thickness of the primary tumor cannot be determined with certainty.
Contraindications to SLNB include histologically confirmed metastatic LN and prior wide local excision [12]. The existence of nodal disease can cause false-negative results, so an ultrasound of the LN prior to performing the SLNB is recommended. Prior wide local excision is a relative contraindication, because it can alter the lymphatic drainage from the primary tumor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree