4
Malignant Spinal Cord Compression
Randy Li-Hung Wei and Kavita V. Dharmarajan
INTRODUCTION
Epidemiology
Malignant spinal cord compression (MSCC) occurs when a soft-tissue tumor or pathological fracture of the spinal column impinges on the spinal cord or its vascular supply. Diagnosis of MSCC occurs in 2.5% to 10% of cancer patients and requires prompt diagnosis and intervention to maximize the chances for preserving neurologic function.1 More than 95% of MSCC are extramedullary and most common secondary to the anterior portion of the vertebral column. Tumor-related back pain is the most common presenting symptom with 83% to 95% of patients with this symptom at presentation.2 This type of pain is usually worse at night or early morning, and improves with activity during the day. The pain improves with a trial of low-dose steroids (i.e., dexamethasone 12-mg daily).3 Other common symptoms are lower extremity weakness, sensory deficits, and loss of autonomic function (bladder and bowel control).
Diagnosis and Management
In patients with a history of cancer, any sensory or motor changes should be immediately concerning for MSCC, since an earlier diagnosis results in improved treatment outcomes. First, a detailed history and focused physical neurological examination is required to document the progression, duration, and extent of sensory or motor loss.
Secondly, an MRI of the spine with contrast of affected area is the most sensitive and specific modality for the diagnosis of MSCC. Given concerns for skip lesions in 10% of patients, an MRI should be performed for the entire spine.4 If the patient has not had any recent imaging or has a newly diagnosed MSCC, then a patient should undergo a CT of the chest, abdomen, and pelvis because the majority of patients with MSCC have visceral and skeletal metastasis. Thirdly, an urgent surgical evaluation of the patient should be obtained, to assess spinal instability and possible surgical debulking and stabilization. The spinal instability neoplastic score (SINS) is a standardized and validated classification system to assess tumor-related instability that includes six individual component scores: spine location, pain, lesion bone quality, radiographic spinal alignment, vertebral body collapse, and posterolateral involvement of spinal elements.5 A SINS score of 0 to 6 denotes stability, 7 to 12 denotes possibly impending, and 13 to 18 denotes instability. Surgical consult is warranted for patients with scores of 7 or greater (Table 4.1).
Treatment requires a multidisciplinary approach. High-dose corticosteroids should be started immediately based on a clinical diagnosis and steroids should not be delayed until diagnostic imaging has been performed. An initial dose of 16-mg intravenous (IV) dexamethasone followed by 4-mg dexamethasone every 4 hours provides immediate improvement in pain and possibly neurologic function. Patients should also be started on a proton pump inhibitor for gastrointestinal prophylaxis. Within 24 to 48 hours, surgery or radiation therapy (RT) should be initiated to maximize the chance of maintaining neurologic function.6,7 A randomized trial suggested that surgery plus postoperative RT for patients with sufficient performance status and prognosis improves neurologic recovery and survival when compared to patients treated with RT alone.8 Surgery should be strongly considered in patients with spinal instability, with compression caused by a retropulsed bone fragment, who have received RT in the same location in the past, and when histologic evaluation is needed to determine the nature of a tumor arising at an unknown primary site. Factors related to the patient (age, performance status, comorbid disease, reluctance to undergo surgery) or the disease (visceral metastases, multiple levels of spinal cord compression, presence of symptoms for more than 72 hours) may reduce the likelihood that the patient will undergo surgery. In patients greater than 65 years old, RT alone may be equally as efficacious as surgery plus postoperative RT.9,10
Radiation Therapy and Dose Fractionation
The optimal dose and fractionation regimen for metastatic epidural spinal cord compression is unknown and must factor in a patient’s prognosis, tumor histology, efficacy, and dose tolerance of the spinal cord.
Many clinicians employ a fractionated courses of radiation to 30 Gy in 10 fractions or 20 Gy in 5 fractions with the rationale that these doses are sufficiently high to provide a good initial and durable response.11 However, two prospective randomized studies failed to show a difference in pain control or ambulation in patients treated with 16 Gy in 2 fractions versus 30 Gy in 10 fractions or 16 Gy in 2 fractions versus a single 8 Gy dose.12,13 Two other studies suggested no difference in improving ambulation, but some improvement in local control when longer courses are given to patients with MSCC.14,15 While any of the studied fractionation schemes (30 Gy in 10 fractions, 16 Gy in 2 fractions, and 8 Gy in 1 fraction) are reasonable for the primary treatment of MSCC, the shorter courses may be more appropriate for those with poor overall prognoses while those with a better prognosis may have a greater benefit with longer courses of RT. The optimal dose for postoperative RT is also unknown, though 30 Gy in 10 fractions is commonly used in the United States.
TABLE 4.1 SINS Component and Numerical Score. A SINS Score of 0–6 Denotes Stability, 7–12 Denotes Possibly Impending, and 13–18 Denotes Instability5
SINS Component | Score |
Location |
|
Junctional (occiput–C2, C7–T2, T11–L1, L5–S1) | 3 |
Mobile spine (C3–C6, L2–L4) | 2 |
Semirigid (T3–T10) | 1 |
Rigid (S2–S5) | 0 |
Pain |
|
Yes | 3 |
Occasional pain but not mechanical | 1 |
Pain-free lesion | 0 |
Bone lesions |
|
Lytic | 2 |
Mixed (lytic/blastic) | 1 |
Blastic | 0 |
Radiographic spinal alignment |
|
Subluxation/translation present | 4 |
Kyphosis/scoliosis | 2 |
Normal alignment | 0 |
Vertebral body collapse |
|
>50% collapse | 3 |
<50% collapse | 2 |
No collapse with >50% body involved | 1 |
None of the above | 0 |
Posterolateral involvement of spinal elements |
|
Bilateral | 3 |
Unilateral | 1 |
None of the above | 0 |
The role for stereotactic body radiation therapy (SBRT) in the treatment of MSCC is unclear and currently under investigation. There are no randomized control trials demonstrating equivalence or superiority to three-dimensional (3D) conformal radiation therapy (CRT), though early results are promising. Prospective randomized data will help define the best uses of this technology.16 Routine use should be avoided until sufficient evidence justifies the substantive increase in cost relative to standard radiation therapy. For patients who require urgent RT, the complexity of treatment planning, dependence on meticulous body immobilization, and the potential for increased side effects including myelitis, dermatitis, and vertebral compression fractures with high dose per fraction make this modality of radiation treatment challenging and less practical.17–19 Though proton therapy has dosimetric advantages over photon radiation, it is not used for palliation of MSCC (Personal communication with Dr. Anita Mahajan).
TREATMENT PLANNING
Target Delineation
If possible, patients should undergo a CT simulation scan for treatment planning. Patients should be in a supine position with appropriate immobilization. Though many use a vac-fix, the potential need to treat adjacent areas and precisely recreate prior RT fields to minimize or eliminate overlap may argue for use of commercially available immobilization devices or a simple headrest and bolster. In addition, some patients may require prone simulation due to pain. If a recent MRI is available, the MRI should be fused with the CT simulation scan to improve target delineation. Both CT and MRI can be used to determine the depth of the vertebral surface of the cord beneath the skin surface. If a CT simulation is unavailable, fluoroscopic simulation can be performed. In this setting, the use of a wire may help to delineate the skin surface to ensure the distance to the posterior surface of vertebral bodies on a lateral radiograph.
The gross tumor volume (GTV) is best seen as the T1-enhancing abnormality or the nonenhancing tumor on T2 or fluid-attenuated inversion recovery (FLAIR) images. If there is no residual tumor after surgical resection, the GTV is defined as the resection cavity. Surrounding edema is not included in the GTV. The clinical target volume (CTV) includes the T2 and FLAIR abnormality, and clinical suspicion for microscopic disease. The width of the posterior fields should encompass the spinal canal with a 1- to 1.5-cm margin. The CTV should also include one vertebral level above and below the GTV to prevent marginal recurrence. The planning target volume (PTV) margins should account for daily variations in patient setup, and potential mass effect from cord edema.
If a patient is being treated urgently a clinical setup can be performed for the first treatment fraction. Using lateral kilovolt (kV) imaging, the depth beyond the mass can be calculated, and the patient can be treated in a source-axis distance (SAD) setup.
Treatment Techniques
Tumors in the cervical spine are often treated with opposed laterals. This technique minimizes radiation dose and consequent side effects within the oral cavity and pharyngeal structures. Tumors in the cervical-thoracic region can be treated with split beam approaches with opposed lateral fields to the upper spine, and a single posterior to anterior (PA) field for the area of the spine below with careful matching of fields. Patients with thoracic spine tumors can be treated with PA fields, opposed anterior to posterior (AP)–PA beams preferentially weighted, or opposed lateral beams. In the lumbar region, a four-field approach using AP–PA and opposed laterals can be used to reduce dose to the kidneys.
If using two-dimensional (2D) or 3D-CRT, fields should extend one vertebral body above and one vertebral body below the MSCC. This ensures that the area of concern is fully covered as isodose lines bow in at the field edges. To facilitate future treatment plans, the top of the radiation field should match the top of the vertebrae to facilitate future treatment plans, and consideration should be given to the use of a half-beam block. Treatment plans should have a reasonably homogenous dose distribution; this can be achieved by a mix of low-energy and high-energy photons and unequal beam weighting.
Laterals for MSCC of the upper cervical (C) spine
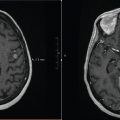
Tumors involving the upper cervical spine can be treated with opposed lateral fields to avoid unnecessary radiation to the oral cavity and hypopharynx (Figure 4.1). However, if the lesion is too inferior, then the shoulders may block the lateral fields. A PA field would be most appropriate in this case.
Single PA field thoracic (T) spine
Figure 4.2 depicts the isodose curves used to treat a 78-year-old man with metastatic prostate cancer who had bone lesions involving the thoracic (T) spine at T2 through T7 with extramedullary extension into the spinal column. A single PA beam was used to encompass the gross disease seen on the planning CT scan. The 100% isodose line encompasses the entire spinal canal. The fields extended laterally to encompass the width of the vertebral body. The sagittal view shows that the 100% isodose line extends anteriorly to encompass the spinal canal.

FIGURE 4.1 Opposed lateral beams for the treatment of the upper cervical spine.
Single PA field lumbar (L) spine
Figure 4.3 depicts the treatment plan for a 58-year-old male with metastatic renal cell carcinoma with posterior epidural tumor invading into the cauda equina. A single 6-megavolt (MV) PA field was used to encompass the gross disease that was encroaching posteriorly through the lamina and into the vertebral foramen. The 100% isodose line covers the anterior edge of the vertebral foramen.
AP–PA treatment of the lumbar (L) spine
Tumors of the lumbar region may require opposed AP–PA fields because of the lumbar curvature and the deep location of the vertebral canal near the midline of the abdomen. Depending on the depth of coverage, a single PA field may result in high dose to the subcutaneous tissue. In Figure 4.4, a 62-year-old male with metastatic bladder cancer has metastatic disease invading into the anterior vertebral body. A parallel-opposed field was used to create a radiation treatment plan with a homogenous dose covering the disease.
Intensity-modulated or volumetric-modulated radiation therapy treatment of the entire cervical spine
Figure 4.5 depicts the treatment of a 47-year-old woman with metastatic breast cancer involving the cervical spine at C2 to C7. Since the patient’s shoulders would block parallel-opposed lateral fields, an intensity-modulated RT or volumetric-modulated arc therapy (IMRT/VMAT) plan was used to treat the spine and reduce esophagitis by limiting the exit dose. Since esophagitis typically lasts only for a few days and can be managed conservatively, a single PA field is simpler and more cost effective.
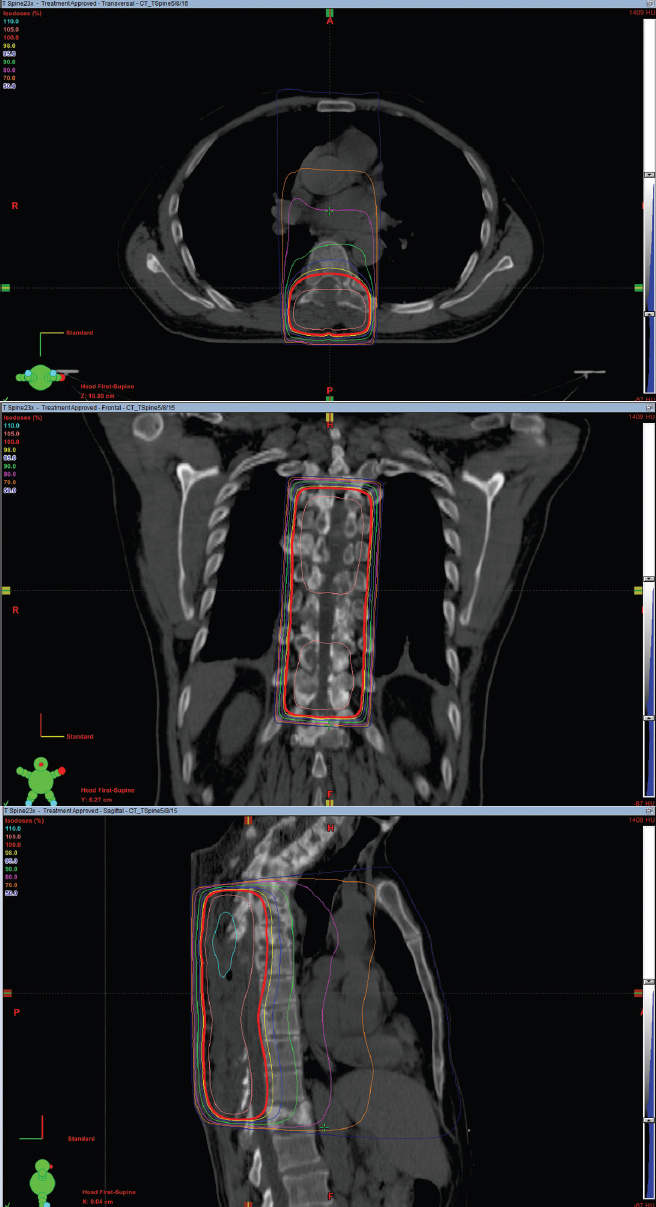
FIGURE 4.2 Axial, coronal, and sagittal images depicting the isodose lines for a single AP beam used to treat vertebral bodies T2 to T7.

FIGURE 4.3 Axial and sagittal images depicting the isodose lines for a single PA beam used to treat the lumbar spine.
Reirradiation for infield recurrences
Advances in systemic therapy have improved overall survival with patients with metastatic cancer. As a result, radiation oncologists encounter patients with recurrent or progressive spinal metastases causing spinal cord compression. It is important to fuse previous radiation treatment fields on current planning CT scan, so as not to exceed a spinal cord dose threshold of 45 Gy. In a retrospective trial, spinal reirradiation with 8 Gy in 1 fraction, 15 Gy in 5 fractions, 20 Gy in 5 fractions, 21 Gy in 7 fractions, and 20 Gy in 10 fractions improved (25%) or stabilized (50%) motor function with no late radiation myelopathy observed. A prospective, randomized trial of reirradiation that included retreatment of the spine demonstrated that 20 Gy in 5 or 8 fractions was equivalent to 8 Gy in a single fraction for uncomplicated bone metastasis. Since 8 Gy in 1 fraction is more cost effective, noninferior, and less toxic, it is the preferred reirradiation regimen. The trial included patients treated with specific prior regimens and the inclusion criteria should be considered when reirradiating the spine. Though this trial does not directly apply to patients with spinal cord compression, it does shed light on spinal cord tolerance to radiation.20 Reirradiation was safe and effective when the cumulative biological effective dose (BED) was less than 120 Gy (alpha/beta of 2).21 SBRT as a primary treatment for metastatic epidural spinal cord compression is feasible but there is a lack of long-term outcomes compared to surgical intervention and/or external beam radiation therapy.22

FIGURE 4.4 Sagittal and axial images depicting AP–PA treatment of the lumbar spine.
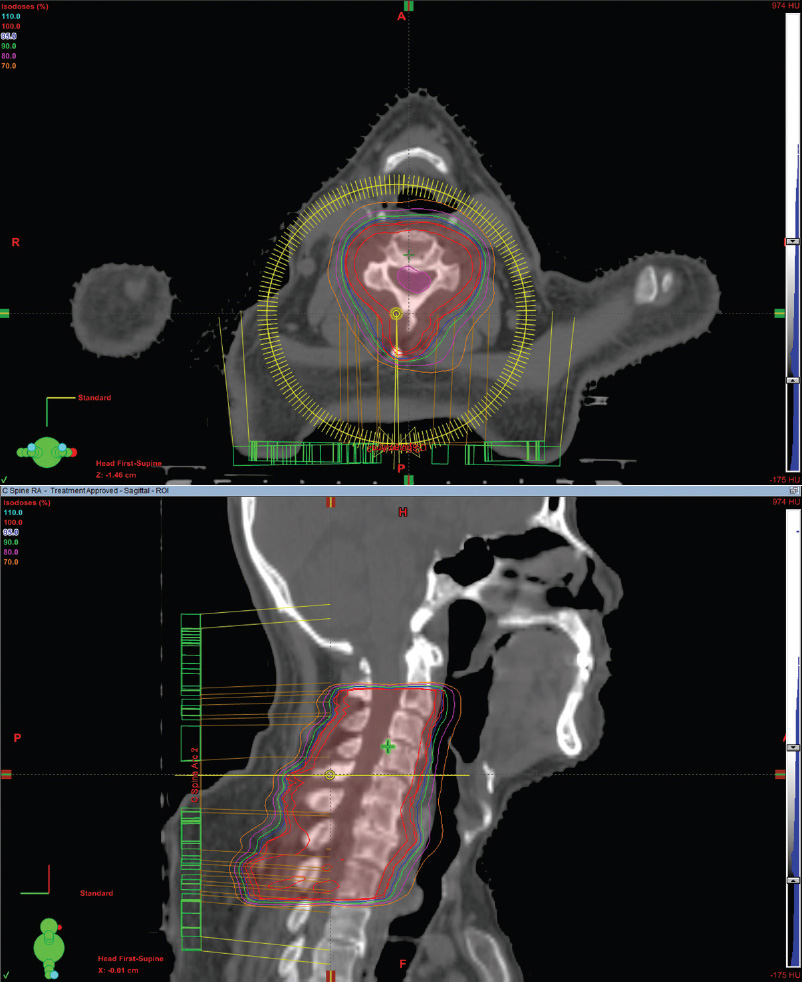
FIGURE 4.5 Axial and sagittal images of a single VMAT arc plan to treat vertebral bodies C2 to C7.
Follow-Up
After completion of RT, follow-up is important in order to assess for changes in pain, motor function, and sensation. There are no studies or guidelines addressing follow-up of treatment for MSCC. However, if pain has not improved within 2 weeks, the physician will need to prescribe or increase steroids, try other classes of analgesics, or reevaluate for concern for disease progression with new radiologic imaging.
Acute and Late Effects
Acute side effects include dermatitis, pain flare, and nausea. The most common acute effect is grade 1 to 2 dermatitis over the treated area. Patients can use petroleum-based products to minimize dryness and erythema. Additionally, pain flare along the spinal cord typically occurs within 1 to 4 days after radiotherapy. Prophylactic use of 8-mg dexamethasone has been shown to reduce episodes of pain flare by 8% compared to placebo.23 Lastly, patients may experience nausea and diarrhea from low-dose radiation in the intestine. Antiemetics, such as 8 mg of ondansetron, are given prior to the dose of radiation to prevent or reduce the development of radiation-induced nausea when the stomach or intestines are in the radiation fields.
In 2 to 6 months, the patient may develop a reversible myelopathy secondary to transient demyelination characterized by shock-like radiation sensations to extremities when the neck is flexed (L’Hermitte’s sign). Symptoms are self-limiting and no therapy is required. If symptomatic, a trial of glucocorticoids can be prescribed. If the patient does not respond, care must be taken to taper off the glucocorticoids since chronic steroid use can result in steroid myopathy.
Rarely, chronic progressive myelopathy is a late complication of radiation that is progressive and chronic with no treatment available. Tumor progression may also cause myelitis and retreatment can be considered, taking into account the spinal cord or cauda equina tolerance and prior delivered dose.24 In pediatric patients, the risk of a secondary neoplasia, spine deformity, and height loss following radiation is significant. Treatment plans should generally include the entire vertebral body to reduce asymmetric growth.
CLINICAL PEARLS