Fig. 2.1
TARGIT impairs the proliferative activity of wound fluids from breast cancer patients. This figure shows the colony formation assay. In (a), MCF-7 breast cancer cells were included in a 3D Matrigel matrix and incubated in medium supplemented with 2 % FBS (control) or wound fluids from untreated or TARGIT-treated breast cancer patients as indicated. Only wound fluids from untreated patients were able to stimulate MCF-7 cell growth in 3D matrix. (b) Depicts the areas of representative colonies formed by MCF-7 cells in the presence of the indicated stimuli (all used at 2 % concentration). The larger size of colonies formed in the presence of wound fluids from untreated patients is evident. Pre Serum indicates the serum collected from untreated or TARGIT-treated women the day before surgery
We have also demonstrated that wound fluids act as chemo-attractants for breast carcinoma cells, which again provides a possible explanation as to why local recurrences peak between 2 and 3 years after primary surgery at the site of surgery (Baum et al. 2005). Indeed, we have provided the first formal demonstration that wound fluids harvested from breast cancer patients who have undergone wide local tumour excision stimulate breast cancer cell motility, invasion and growth in three-dimensional contexts (Belletti et al. 2008). Thus, the wound fluid may stimulate the growth of any residual tumour cells and/or attract breast cancer cells at the site of surgery, suggesting an additional molecular and biological explanation for the high local recurrence rate of breast cancer.
We can therefore speculate that surgical excision of the cancer is certainly beneficial but the act of surgery may have harmful effects (Demicheli et al. 2001; Baum et al. 2005) and we should strive to understand and reduce these. We have demonstrated that TARGIT almost completely abrogates the stimulatory effects of surgical wound fluid on cancer cells in vitro (Fig. 2.2), suggesting that it may confer more benefits than those expected from the tumoricidal effect of radiotherapy.
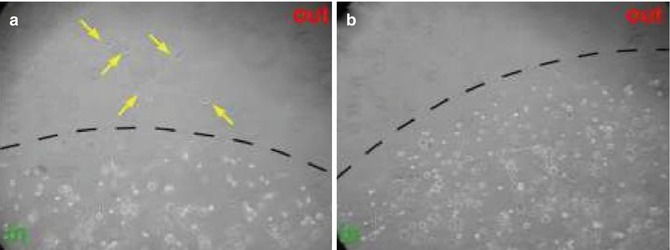

Fig. 2.2
TARGIT decreases the chemo-attractant activity of wound fluids from breast cancer patients. This figure shows an evasion assay. MDA-MB-231 highly metastatic breast cancer cells were included in a 3D Matrigel matrix and incubated in medium supplemented with 5 % wound fluids from untreated (a) or TARGIT-treated (b) breast cancer patients. Only cells incubated with wound fluids from untreated patients were able to evade the matrix (yellow arrows) in response to chemo-attractant stimuli
Importantly, our observations are in line with the clinical evidence demonstrating that TARGIT is able to control local recurrences as well as EBRT when used alone in selected patients (Vaidya et al. 2010), but also results in better local control compared with that obtained with the EBRT boost when used as a tumour bed boost in unselected breast cancer patients (Vaidya et al. 2011).
The latter clinical observation suggests that the beneficial effect of TARGIT could have contributed in achieving such a low rate of recurrence not only through better cell killing (probably the main mechanism of action) but also by modifying the wound micro-environment, making it less favourable for cancer cell growth and invasion. Of course, while our studies do not provide proof for the superiority of IORT, they do provide a biological rationale for that possibility. As a corollary we can also speculate that the effects of radiotherapy may be completely different when radiation is applied to a wounded tissue as compared with application to an already repaired breast, as in the case of EBRT in breast cancer therapy. To prove this hypothesis we generated two different mouse models of breast radiotherapy (Fig. 2.3) that indeed confirmed our speculation (manuscript in preparation).
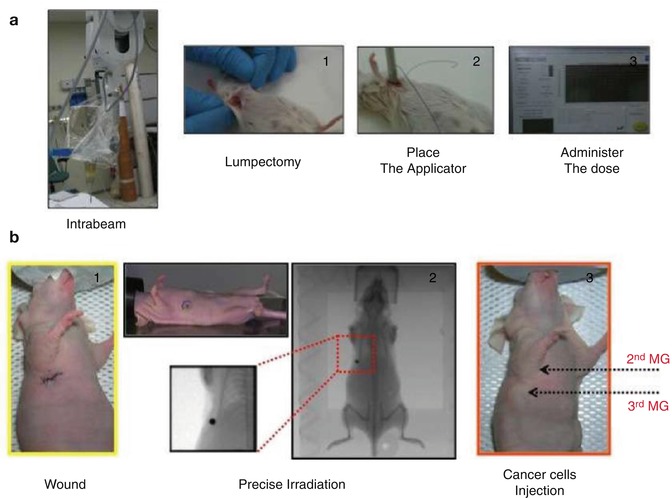

Fig. 2.3
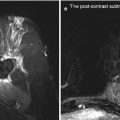
Mouse models of TARGIT and precise irradiation. In (a) the INTRABEAM apparatus applied to the mouse breast irradiation is shown. Briefly, after anaesthesia, female SV129 mice underwent lumpectomy and then the INTRABEAM applicator was placed in the wounded tissue. Delivered doses (up to 8 Gy at 2 mm) could be delivered with two different applicators in order to vary the time of exposure. Wounded and irradiated tissues could be studied after different time points from irradiation to gain new insights into the biology of the irradiated wounded tissues. In (b) the precise irradiator was used as a model of wounded and undamaged breast. Briefly, the second mammary gland of nude female mice was wounded or not (1) and then the irradiation of both second and third mammary glands was performed using an area as small as 2 × 2 mm2 (2). Breast cancer cells were then injected in the third mammary gland (3) and tumour growth followed over time. In this manner it was possible to identify the specific effects of radiation on the wound-induced growth of breast cancer cells in vivo
From a biochemical point of view, we demonstrated by a proteomic analysis on 174 known cytokines that TARGIT is able to modify in vivo in humans the levels of several factors involved in the control of cell growth motility and metastasis formation, such as IL-6, IL-8, HGF, UPA, leptin and RANTES (Belletti et al. 2008). Moreover, in accordance with previous observations on the effects of radiotherapy on modification of cytokine expression in humans and animal models (Yamazaki et al. 2005; Jones et al. 1999; Büttner et al. 1997), we observed a specific increase in IL-5 and IL-4 following TARGIT that could also reflect a different immune response in the local micro-environment. Indeed, the modification of immune response by radiotherapy has recently been proposed (Formenti and Demaria 2008; Formenti and Demaria 2009).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree