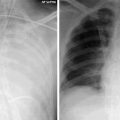
Fig. 23.1
ECG-gated Cardiac CT on a 45 year-old male patient with history of childhood road traffic accident shows an incidental aortic pseudoaneurysm (black star), presumed secondary to previous undetected transection. This is causing severe compression of the left main bronchus (block arrow)
As obtaining a secure airway is vital, prompt diagnosis and early intervention are crucial.
In the presence of severe symptoms of cardiorespiratory compression such as, positional dyspnea, stridor, orthopnea, syncope, and SVC syndrome the induction of general anesthesia may be fatal. In high-risk patients with mediastinal mass, irreversible cardiorespiratory collapse can occur with as little as the use of a sedative or simply by putting the patient in a supine position. It is also possible that tracheobronchomalacia due to prolonged compression by a mediastinal mass may potentiate the airway collapse, especially after tracheal extubation (Fig. 23.2). Therefore, airway management in patients with large mediastinal masses with or without direct airway compromise poses a difficult challenge to the intensivists.
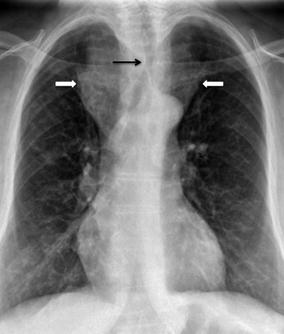

Fig. 23.2
A 65 year-old female with a large anterior mediastinal mass secondary to retrosternal thyroid goiter (white block arrows) and resultant compression of the trachea (black arrow)
Venous and Arterial Compromise
Tumors may invade the heart, pericardium and great vessels by means of lymphatic or hematogenous dissemination, local extension, or, more uncommonly, via the transvenous route. Tumors that are most likely to involve the heart and pericardium include cancers of the lung, breast cancer, lymphoma, and melanoma. Metastases to the heart and pericardium can manifest as a lung mass or mediastinal mass with direct invasion of adjacent structures such as the heart, as a central mass extending into the left atrium via the pulmonary veins, as pericardial effusion and nodularity, or as myocardial lesions.
Profound hypoxia may also occur due to compression of great mediastinal vessels in the presence of patent airways. Severe symptoms of cardiorespiratory compromise can be due to right ventricular outflow tract or pulmonary artery compression.
The SVC is vulnerable to extrinsic compression and obstruction because it is thinwalled, its intravascular pressure is low and its anatomical position which is confined by lymph nodes and other rigid structures (Fig. 23.3).
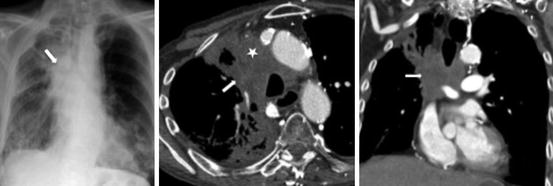

Fig. 23.3
A 55-year old female with small cell lung cancer. CXR (far left) shows a large right-sided mediastinal mass (block arrow) with obliteration of right paratracheal stripe. Axial (middle) and Coronal CT images demonstrate the large necrotic mediastinal mass (block arrow) with invasion and occlusion of the superior vena cava (white star)
Hemoptysis
This serious, potentially life-threatening complication can be due to airway erosion by tumors or pulmonary hemorrhage. Pulmonary artery injury or aneurysm formation secondary to Swan Ganz catheter manipulation is one of the uncommon iatrogenic causes of hemoptysis.
Diagnostic studies for massive hemoptysis include CXR, bronchoscopy, contrast enhanced CT of the chest and the gold standard ‘selective angiography’. In patients with massive hemoptysis, the work-up is usually performed both to find the cause of the bleeding and to localize its site.
Fibreoptic bronchoscopy (FOB) is readily available and can be performed in patients too unstable for transfer to the CT scanner. Bronchoscopy has been considered to be a primary method for diagnosis and localization of hemoptysis. FOB has been proven to be efficacious in evaluating central bronchial lesions. A vasoactive drug can be applied locally during bronchoscopy to control bleeding.
Bronchoscopy has some disadvantages in the diagnosis and localization of massive, active hemoptysis. It can be difficult to localize the bleeding site because of excessive blood in the bronchi and endobronchial therapies are not effective in most cases of massive hemoptysis. The risks of bronchoscopy include possible airway compromise, delay in definitive treatment, and hypoxemia.
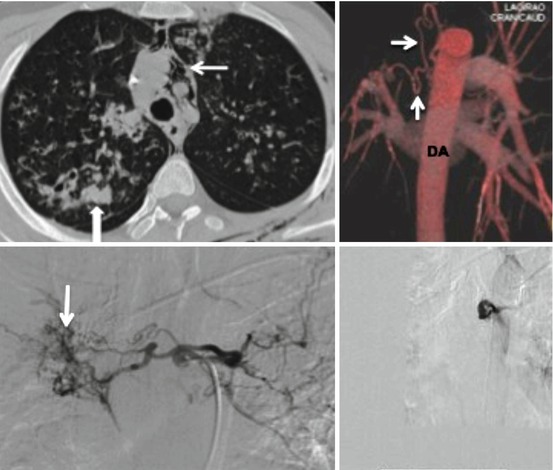
Contrast CT has been shown to be of considerable value in diagnosing bronchiectasis, vascular lesions, eroding aspergilloma, and bronchogenic carcinoma in patients with massive hemoptysis (Fig. 23.4). A major advantage of CT over other imaging modalities is the ability to exclude other causes of bleeding, i.e. mimics of hemoptysis, from nasopharynx and gastrointestinal tract and provide a vascular road map. The argument for performing a CT prior to bronchoscopy or embolization is to localize the pulmonary lobe in which bleeding is suspected and identify enlarged bronchial or non-bronchial collateral vessels (Fig. 23.5). Knowing the potential site for bleeding permits intervention such as embolization to be focused and performed in a safe and efficient manner. CT and bronchoscopy are not competitive but complementary tools for assessing and managing patients with hemoptysis, and indeed, the combined use of bronchoscopy and CT does yield the best results. Angiographic findings in massive hemoptysis include neovascularity, hypertrophic and tortuous bronchial arteries, shunting into the pulmonary artery or vein, bronchial artery aneurysm, and extravasation of contrast medium. Determination of which arteries are to be embolized should be based on a combination of CT, bronchoscopy, and angiographic findings together with clinical correlation.
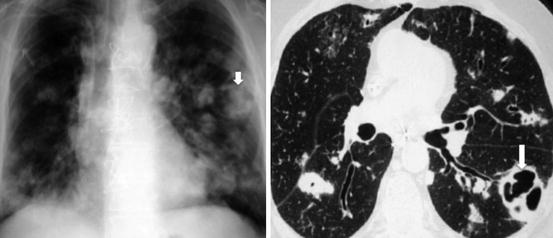

Fig. 23.4
CXR and CT on a 30 year-old male intravenous drug abuser with multiple bilateral septic infarcts. The largest of the cavities in the left lung (block arrow) has intracavitary soft tissue and air, proven to be aspergillus