The mediastinum is the most common location for intrathoracic masses in children. Mediastinal masses in pediatric patients are composed of a heterogeneous group of lesions ranging from benign, asymptomatic, and incidentally detected lesions to malignant, symptomatic, and potentially life-threatening masses. Evaluation of the patient with a mediastinal mass often requires a multidisciplinary approach, with imaging playing a critical role in detection, characterization, and monitoring of disease.
How Do You Differentiate Mediastinal From Pulmonary Parenchymal Masses?
On radiographs, it is important to differentiate lesions arising from the mediastinum from primary pulmonary parenchymal abnormalities. Mediastinal masses will cause obtuse angles at their interface with aerated lung, whereas intrapulmonary masses will result in acute angles between the mass and adjacent lung. Primary lung lesions may contain air bronchograms, a finding that is not present in mediastinal masses. Masses within the mediastinum may cause shifting or obliteration of junctional lines and the azygoesophageal recess.
How Is the Mediastinum Subdivided?
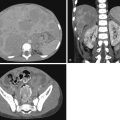
The mediastinum is a compartment of the thorax located in between the two pleural cavities, posterior to the sternum and anterior to the vertebral column. The superior margin is defined by the thoracic inlet, and the inferior boundary is the diaphragm. The mediastinum is divided into three parts: anterior, middle, and posterior. Because there are no fascial planes anatomically dividing the different sections of the mediastinum, the boundaries of the subdivisions are arbitrarily based on anatomic landmarks delineated on a lateral chest radiograph ( Fig. 2.1 ). Knowledge of the anatomic structures occupying each compartment will help guide a differential diagnosis when evaluating a mediastinal mass ( Table 2.1 ). In addition, the differential can also be narrowed by using the imaging characteristics to suggest tissue type ( Table 2.2 ).

| Anterior mediastinal masses |
|
| Middle mediastinal masses |
|
| Posterior mediastinal masses |
|
| Lesion Characteristics | Differential Diagnosis |
|---|---|
| Fat containing |
|
| Cystic |
|
| Calcifications |
|
| Homogenous |
|
| Heterogenous |
|
What Normal Structures Occupy Each Compartment of the Mediastinum?
The anterior mediastinum, also known as the perivascular space, is the space posterior to the sternum and anterior to the heart and brachiocephalic vessels. Its contents include the thymus, lymph nodes, and fat. The middle mediastinum, or vascular space, is bordered by the pericardial reflections. The contents of the middle mediastinum include the heart, great vessels, trachea, central bronchi, lymph nodes, and fat. The posterior mediastinal compartment, or postvascular space, lies behind the pericardial reflection and extends to the posterior chest wall. The contents include the descending thoracic aorta, esophagus, thoracic duct, azygous and hemiazygous veins, sympathetic nerves, and lymph nodes.
Anterior Mediastinum
What Is the Normal Appearance of the Thymus on Imaging Studies?
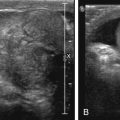
The thymus is an encapsulated bilobed, soft organ within the anterior mediastinum that functions to produce T cells. The thymus is largest in size at birth, in relation to the patient’s body size; however, it is largest in absolute size just before puberty. After puberty, it begins to involute and progressively becomes replaced with fat. On radiographs, it is a prominent soft tissue density in the anterior mediastinum, visible until about 3 years of age. The left lobe is usually larger than the right. On the lateral radiograph, the soft tissue density usually occupies the anterior mediastinum abutting the upper cardiac border. On the frontal projection, it manifests as widening of the mediastinum often with a lobulated margin (wave sign) as a result of indentation from the ribs ( Fig. 2.2 ). Often the right lobe of the thymus has a flat inferior margin at its interface with the minor fissure (sail sign) ( Fig. 2.3 ). A characteristic notch may be seen at the junction of the thymus and cardiac silhouette ( Fig. 2.4 ). At fluoroscopy the thymic shadow should widen in expiration and narrow with inspiration because of its pliability. The thymus has a characteristic appearance on ultrasound with short linear echogenic structures corresponding to connective tissue septae ( Fig. 2.5 ). On computed tomography (CT) the thymus occupies the anterior mediastinum and is typically homogenous in attenuation and does not cause mass effect on adjacent structures ( Fig. 2.6 ). On magnetic resonance (MR) imaging (MRI), it is slightly hyperintense on T1-weighted imaging (T1WI) with respect to skeletal muscle and homogeneously mildly hyperintense in signal on T2-weighted imaging (T2WI) ( Fig. 2.7 ). Familiarity of the MR appearance of the thymus is helpful when evaluating for ectopic or aberrant thymus, which should follow thymus signal characteristics on all pulse sequences ( Fig. 2.8 ).







Where Can Ectopic Thymus Implant?
Embryologically the thymus is derived from the third pharyngeal pouch, with minor rudimentary portions derived from the fourth pharyngeal pouch. Beginning in the eighth week of gestation, the primordial thymus descends from the level of the pharynx inferiorly to the mediastinum along the thymopharyngeal duct, deep to the thyroid gland and sternocleidomastoid and along the carotid sheath. During its descent, remnant thymic tissue may implant along the path of migration, which may result in a neck or mediastinal mass. In the rare variant retrocaval thymus, an aberrant portion of the gland may extend posteriorly between the superior vena cava and great vessels, mimicking a neurogenic tumor. Among reported cases of posterior extension of the thymus, there is a right-sided and male predominance. On cross-sectional imaging, the aberrant thymus should appear identical to the thymus and is usually in continuity with the normally located thymus. Management is typically nonoperative; however, on occasion a large ectopic thymus can cause symptoms as a result of compression of nearby structures prompting surgical resection.
Thymic Hyperplasia
Thymic hyperplasia is a rebound phenomenon seen in patients recovering from thymic atrophy, usually caused by stressors such as chemotherapy, corticosteroids, or radiation therapy. Six months after the cessation of chemotherapy is the average time interval that thymic rebound is observed in pediatric patients. However, it may be seen in just a few weeks if the child was being treated with steroid therapy alone and has been reported as late as 5 years posttreatment. The thymus may rebound to a size even larger than its expected normal size ( Fig. 2.9 ). Differentiating thymic hyperplasia from recurrent neoplasm can be problematic. On cross-sectional imaging, thymic hyperplasia appears as enlargement of the thymus with attenuation at CT and signal intensity at MRI equivalent to that of a normal thymus. In adolescent pediatric patients (>15 years of age), chemical shift MRI can be used to reliably differentiate thymic hyperplasia from neoplasm because of the presence of intracellular lipid in the hyperplastic thymic gland. In thymic hyperplasia, loss of signal on opposed phase images is diagnostic of hyperplasia, a finding not present with neoplasm. In patients younger than 15 years, chemical shift imaging may not be helpful because the physiological process of fatty infiltration of the thymus may not have commenced.

Thymic lymphoid hyperplasia refers to an increase in the number of lymphoid follicles, which is associated with myasthenia gravis and other autoimmune disorders, such as systemic lupus erythematosus, rheumatoid arthritis, scleroderma, and Graves disease. The morphological imaging characteristics of thymic lymphoid hyperplasia and true hyperplasia are indistinguishable; however, lymphoid hyperplasia may be higher in attenuation on CT compared with true hyperplasia.
Thymic Cyst
Thymic cysts are benign cystic lesions arising from the thymus that can be either congenital or acquired. They are typically incidentally identified on cross-sectional imaging performed for unrelated reasons. Although most patients are asymptomatic, there is an association with autoimmune disorders that is reported in up to one-third of patients with intrathymic cysts. Congenital thymic cysts are usually unilocular and contain simple fluid and a thin, imperceptible wall. Acquired thymic cysts, in contrast, may be multilocular and contain complex fluid as a result of hemorrhage or infection. Conditions associated with acquired thymic cysts include Hodgkin lymphoma, thymic tumors, thymic hyperplasia, chest trauma, and HIV infection. On CT, thymic cysts are typically midline, round or oval, well circumscribed, and can be either hypodense or hyperdense on CT depending on the protein content. Calcium and fat attenuation should be absent, distinguishing features from a germ cell tumor (GCT). At MRI, thymic cysts are hyperintense on T2WI, isointense or hypointense to skeletal muscle on T1WI, and thin, peripheral enhancement on postcontrast sequences.
Thymic Epithelial Tumors: Thymoma and Thymic Carcinoma
Thymic epithelial tumors are a group of tumors of epithelial cell origin that arise from the thymus gland and have a variable amount of lymphocytes. In 1999, the World Health Organization (WHO) reclassified thymic epithelial tumors on a continuum as either thymoma (subtypes A, AB, B1, B2, and B3) or thymic carcinoma (type C), based on their morphological appearance and ratio of lymphocytes to epithelial cells. Thymomas demonstrate no overt atypia of the epithelial cells and retain features specific to the normal thymus. Immature, nonneoplastic lymphocytes are present in variable numbers. Thymomas are the most common type of thymic epithelial tumor, accounting for almost half of all anterior mediastinal masses in the adult patient population. In children, however, they are less common and account for only 1% to 2% of all mediastinal masses. Approximately 40% of patients with thymoma present with a paraneoplastic syndrome, most often myasthenia gravis. Notably, 15% of patients with myasthenia have a thymoma.
Histologically, thymic carcinomas exhibit cellular atypia and no longer demonstrate features specific to a normal thymus. They resemble cytological features of carcinomas of other organs. Thymic carcinomas are usually advanced at the time of diagnosis, have a greater recurrence rate than thymomas, and have a worse 5-year survival. Unlike thymoma, the association of autoimmune disease is uncommon.
Noninvasive type A thymomas have been described as well-defined smooth margins, with a round shape, and tend not to have extracapsular extension. Invasive thymoma, however, does extend beyond the confines of the fibrous capsule with local spread to adjacent structures, such as mediastinal structures and the chest wall. Invasive thymoma may spread to the pleura and often recurs after surgical resection.
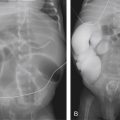
Radiographically, thymomas often appear as anterior mediastinal masses with obliteration of the retrosternal clear space on the lateral projection ( Fig. 2.10A and B). On frontal radiograph, there is contour abnormality of the mediastinum with a smooth lobulated border. Thin peripheral calcification may be present. Pleural nodu larity and pleural effusion may indicate invasive disease. Diaphragmatic elevation is suggestive of phrenic nerve involvement.