Fig. 1
Hepatopulmonary shunt calculation using the values obtained from 99mTc-MAA planar scintigraphy for two different cases (a and b). ROIs are drawn in anterior and posterior planar images covering both lungs and the liver area to be treated
The percentage of LS can be determined from the total counts within ROIs over both lungs and the liver, using the geometrical mean of anterior and posterior thoracic and abdominal planar images.
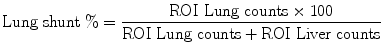
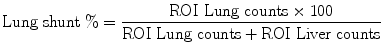
A good correlation exists between the calculation of LS using 99mTc-MAA scintigraphy and the LS calculated using 90Y microsphere images [mean LS with 99mTc MAA: 4.77 ± 2.81, mean LS after treatment: 4.52 ± 2.5 %; coefficient of correlation (r) 0.96], which confirms the ability of 99mTc-MAA to predict the LS of 90Y-microspheres during RE (Jha et al. 2012).
Only recently, the use of SPECT/CT for LS calculation has been proposed to circumvent the limitations of planar images to accurately delineate liver and lung tissues (Yu et al. 2013). Moreover, as planar images lack the 3-D tissue densities required for an adequate attenuation correction of lung and liver tissues, LS calculations based on the use of such images could be overestimated.
The radiation dose (Gy) to the lungs can be predicted by the 99mTc-MAA shunt fraction and the injected activity of the 90Y-microspheres according to the following equation (Leung et al. 1994).
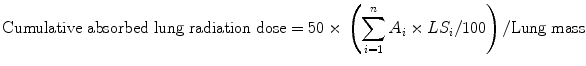 where:
where:
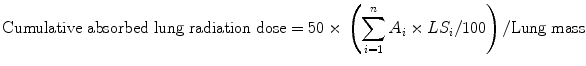
Ai
activity infused (GBq)
LSi
lung shunt fraction during infusion
N
number of infusions,
Lung mass
assumed to be 1 kg.
Previous pre-clinical and clinical studies with 90Y-microspheres demonstrated that the highest tolerable dose to the lungs is 30 Gy for a single injection (Leung et al. 1995; Yorke et al. 2005). In cases where several treatments are administered, the lung dose of radiation is the cumulative absorbed lung radiation dose from all treatments and should not exceed 50 Gy. Depending on the LS value, it might also be necessary to reduce the total administered activity to the liver, or even to contraindicate the use of 90Y RE.
2.3 Extrahepatic Vessels: Unnoticed Collateral Vessels
To perform any therapeutic transarterial procedure in the liver in a safe and efficient manner, it is essential to be acquainted with the hepatic arterial anatomy (Covey et al. 2002). This is particularly important when 90Y-microspheres could be inadvertently deposited in excessive amounts in organs other than the liver, such as the stomach, duodenum, gall bladder, pancreas, mesentery and, to a lesser degree, vascular structures. Serious complications that include GI ulceration, bleeding, gastritis, duodenitis, cholecystitis, pancreatitis, radiation dermatitis and pneumonitis, could occur (Leong et al. 2009; Riaz et al. 2009).
Evaluation of the extrahepatic uptake of 99mTc-MAA using planar scintigraphy analysis can be challenging at times, leading to the misinterpretation of possible extrahepatic side effects. The location of several organs within a relatively small region in the upper abdomen demands the analysis of tomographic images. SPECT and CT or MRI fused images can be employed for the detection of the extrahepatic location of 99mTc-MAA and, thus, detection of the potentially dangerous misplacement of the radiospheres.
As was discussed above, SPECT with integrated low-dose CT increases the sensitivity and specificity of 99mTc-MAA SPECT when detecting extrahepatic arterial shunting. Planar 99mTc-MAA scintigraphy, non-attenuation-corrected SPECT, and SPECT/CT have been reviewed retrospectively for extrahepatic 99mTc-MAA deposition in ninety diagnostic hepatic angiograms obtained from 76 patients with different types of cancer (Ahmadzadehfar et al. 2010). The sensitivity for detecting extrahepatic shunting with planar imaging, SPECT, and SPECT/CT was 32, 41 and 100 %, respectively, while specificity was 98, 98 and 93, respectively. Moreover, therapy planning was changed according to the results of planar imaging, SPECT, and SPECT/CT in 7.8, 8.9 and 29 % of patients, respectively (Ahmadzadehfar et al. 2010). These results have been confirmed recently, suggesting the superiority of SPECT/CT over planar imaging for the identification of digestive, extrahepatic 99mTc-MAA uptake sites, including the gall bladder, which were found in more than one-third of cases (Lenoir et al. 2012).
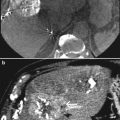
Accordingly, 99mTc-MAA SPECT/CT should now form part of the clinical assessment prior to RE in order to better identify the risk of digestive shunts. In cases of GI uptake on 99mTc-MAA SPECT/CT, a repeated angiographic assessment is mandatory as the source of gastroduodenal flow can frequently be identified and corrected. However, the described series (Lenoir et al. 2012) revealed a high frequency of vascular uptake at the level of portal vein thrombosis, hepatic artery, falciform artery or coil embolisation sites, without any consequences for the therapeutic management of patients. Experts must recognise these uptake sites as possible sources of error (Fig. 2).
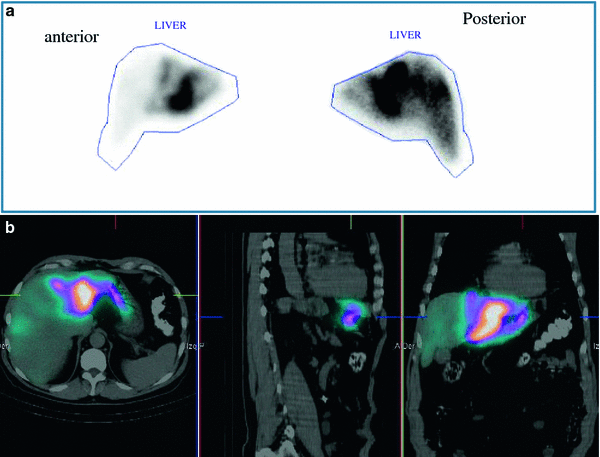

Fig. 2
99mTc-MAA planar scintigraphy (a), and SPECT/CT (b) in a patient with liver metastases from colorectal cancer. Unsuspected extrahepatic visceral shunting in the planar scintigraphy is clearly shown in the fused SPECT/CT images (gastric accumulation)
2.4 Effective Tumour Targeting and Liver Target Volume
RE with 90Y-microspheres is based on delivering a high dose of internal radiation to the tumour while maintaining a safe dose of radiation to sensitive tissues such as the lung and non-tumoural liver. The radiation dose is therefore non-homogeneous, and the precise dose received will vary within the tumour and normal liver parenchyma. For the calculation of the activity that allows the highest dose in the tumour while sparing normal liver tissue, some models have been proposed. One of these is the partition model. This model, which is based on medical internal radiation dosimetry (MIRD), partitions the lungs, tumour and non-tumoural liver into separate compartments for radiation dose modelling.
Assuming that LS and the relative distribution of 99mTc-MAA in the tumoural and non-tumoural liver compartments (tumour targeting or tumour-to-non-tumour ratio, T/N) are similar to that of 90Y-microspheres during subsequent treatment, the activity of 90Y-microspheres to be administered can be estimated using the percentage of LS and T/N determined from the 99mTc-MAA images (Ho et al. 1996).
T/N can be determined from the following equation:
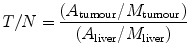 where:
where:
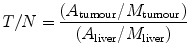
A Tumour
activity in the tumour determined from 99mTc-MAA images
M Tumour
mass of tumour in the liver
A Liver
activity in the normal liver determined from 99mTc-MAA images
M Liver
mass of the normal liver (excluding tumour).
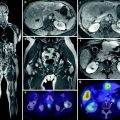
Although no standardised technique has been defined, several methods have been described for the calculation of SPECT/CT-based T/N ratios (Campbell et al. 2009; Flamen et al. 2008; Gulec et al. 2007; Kao et al. 2012). However, European Association Nuclear Medicine procedure guidelines for the treatment of liver cancer with intra-arterial radioactive compounds specify that the T/N ratio cannot be accurately determined unless an attenuation-corrected SPECT scan is used (Giammarile et al. 2011). According to our experience, we calculate the T/N ratio by obtaining the activities of ten identical ROIs drawn on the attenuation-corrected SPECT images obtained by means of SPECT/CT. Five ROIs are of relevant tumour lesions and another five involve relevant non-tumoural areas of the liver. As the number and size are the same for tumoural and non-tumoural tissue, the ratio between the sum of image counts represents an estimation of the T/N ratio. If the tumour is poorly delineated with the CT images obtained from the SPECT/CT acquisition, corregistered SPECT and diagnostic contrast-enhanced CT or MRI fused images should be obtained (Fig. 3).

Fig. 3
Effective tumour targeting between structural lesions (MR or CT) and 99mT-SPECT/CT distribution in a case of hepatocellular carcinoma (a), and liver metastases from colorectal cancer (b)
According to Lau et al. (1994), the tumour targeting (T/N ratio) predicted by the 99mTc-MAA images showed good correlation with the T/N of radiation doses measured with a calibrated beta-probe during open surgery and also with the T/N determined from the liquid scintillation counting of biopsies taken after infusion of the microspheres (Lau et al. 1994). Consequently, we could hypothesise that tumours with high 99mTc-MAA uptake receive a high dose of 90Y-microspheres and, therefore, may respond correspondingly better than tumours demonstrating a low uptake of 99mTc-MAA. However, this hypothesis has not been entirely corroborated. Some authors have not found a significant correlation between tumour 99mTc-MAA uptake and tumour response (Hamami et al. 2009). In this regard, we studied 138 target lesions from a group consisting of 17 hepatocellular carcinoma, 14 colorectal cancer and seven neuroendocrine metastasis patients treated with 90Y RE (Rodríguez-Fraile et al. 2008). The response to RE was based on CT or MRI changes in the maximal diameter observed in each target lesion at 3 and 6 months after RE. In agreement with the previous studies, we did not find any significant correlation between the tumour size changes during the follow-up and the 99mTc-MAA SPECT T/N ratio for each target lesion. In contrast, other authors have shown that the T/N ratio predicts the tumoural response and even the survival (Dancey et al. 2000; Flamen et al. 2008). Using a T/N ratio threshold of unity, Flamen et al. showed that positive and negative predictive values for tumour response prediction were 71 % (17/24) and 87 % (13/15), respectively (Flamen et al. 2008) (Fig. 4).
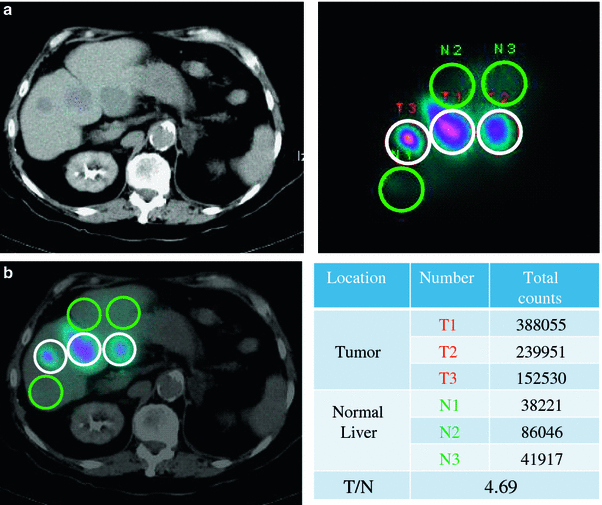

Fig. 4
99mTc-MAA SPECT/CT carried out for T/N calculation during the evaluation work-up of a patient with liver metastases from a neuroendocrine tumour. White circles are located over the liver tumour lesions, and green circles of the same size over the normal liver tissue are shown in the attenuation-corrected SPECT images. Total counts for each region and the calculated TN value are shown in the table
The liver volume that shows an increased uptake after selective injection of 99mTc-MAA into the hepatic artery reflects the vascularised volume to be treated with 90Y-microspheres. For this reason, an important goal of the 99mTc-MAA hepatic perfusion study is to identify the liver volume fed by a selective arterial branch (liver target volume). The calculation of this volume is critical because it directly affects the radiation-absorbed dose estimation. This is especially relevant when planning for selective (lobar) or superselective (segmental or subsegmental) 90Y RE, and in patients with complex anatomical liver vascularisation. 99mTc-MAA SPECT/CT vascularised volume measurement is a more functional and reliable method than current volume calculations made using anatomical images based on CT or MRI data. Moreover, an unexpectedly large volume of target liver, slightly or non-vascularised, was revealed for some patients in the 99mTc-MAA SPECT/CT images (Garin et al. 2011). This is probably due to the existence of microvascular communications between different anatomic segments, most likely via intratumoural arterioportal shunts with low arterial blood flow that went unnoticed on angiography but were identified with 99mTc-MAA SPECT/CT. The existence of an unexpected non-vascularised liver target volume requires the site for delivering 90Y-microspheres to be redefined.
2.5 Conditioning Factors
Some factors can affect the vascular flow of liver tumours and may need to be taken into account when performing the pre-treatment assessment of candidate patients for 90Y RE. Consequently, these factors can also affect the 99mTc-MAA scintigraphy distribution, and should be considered during the evaluation of nuclear medicine procedures.
2.5.1 Prior Treatments
Previously administered treatments are not an excluding factor for hepatic 90Y RE, the only exceptions being prior liver external-beam radiotherapy, and patients with tumour recurrence after liver transplantation (Kennedy et al. 2012).
Exposure to systemic treatments often compromises the hepatic vascular flow, its morphology and neogenesis in the neoplastic tissue and even in the normal parenchyma. All these changes might affect pre-treatment 99mTc-MAA scintigraphy and, therefore, the biodistribution of 90Y-microspheres. In this respect, it is important to note that irinotecan and oxaliplatin can produce sinusoidal obstruction syndrome, and that gemcitabine and 5-FU might increase the risk of liver toxicity. Because of the increased risk of radiation-induced liver disease, concomitant chemotherapy with capecitabine should be discontinued at least for 2 months prior to 90Y RE and must not be prescribed at any time after treatment (Giammarile et al. 2011; Lau et al. 2012; Murthy et al. 2005). Nevertheless, it remains unclear if these drugs represent a contraindication to RE.
Special attention should be paid to anti-angiogenic therapy (bevacizumab, sorafenib). In our experience, patients treated with anti-angiogenic therapeutic drugs show a limited uptake of 99mTc-MAA and poor tumour targeting and, consequently, a very low T/N ratio, which is probably related to the hypoxic conditions created by this therapy. It has been recommended that these drugs be discontinued for at least 8 weeks before evaluation work-up (Ahmadzadehfar et al. 2012).
2.5.2 Vascular Flow Redistribution
The term ‘vascular redistribution’ defines any endovascular technique performed to proximally occlude a major hepatic arterial trunk (lobar or segmental) and thereby allow the perfusion of its distal branches by intrahepatic collaterals. In some cases, the liver lobe has two or more afferent vessels originating from different abdominal trunks (replaced or aberrant arteries), while in others, two or more segmental vessels originate from the right and left hepatic arteries (i.e. segment IV vascularisation). For such cases, the flow redistribution during the pre-treatment angiography, by means of modification to the arterial hemodynamics as well as isolation of the main injection point(s) (one or two), permits to safely administer the 90Y-microspheres to the whole tumoural volume (Bilbao et al. 2010).
To confirm the safety and efficacy of vascular redistribution, 99mTc-MAA SPECT/CT images must show a homogeneous accumulation of MAA over the entire tumoural territory (Kennedy et al. 2009).
3 Treatment Administration and Monitorisation
90Y RE implies the manipulation of radioactive material to ensure the accurate administration of the previously calculated activity of 90Y for each individual. Additionally, the facility in which treatment is administered must be appropriately staffed, be fitted with radiation safety equipment as well as procedures available for waste handling and disposal, and for handling contamination. Personnel must also be able to monitor for accidental contamination and to control its spread. Trained medical staff with supporting physicist and nursing staff should undertake the administration of 90Y-microspheres. Planar scintigraphy, SPECT or PET acquisition, performed a few hours after the administration, isuseful to locate the activity (Giammarile et al. 2011).
3.1 Dose Preparation
Commercially available 90Y-microspheres (SIR-Spheres™, Theraspheres™) are 20–40 μm (ø) particles that emit β-radiation. SIR-Spheres™ are provided in vials with an activity of 3 GBq, while Theraspheres™ are supplied in 6 different activities: 3, 5, 7, 10, 15 and 20 GBq. Consequently, it is necessary to remove the desired activity of 90Y-microspheres from the shipping container and place in the treatment vial for use.
The activity of 90Y-microspheres must be determined by measurement using an appropriate dose calibrator, such as an ion chamber, on arrival or at the time of dose preparation. It is important to note that the dose calibrator is unable to detect β-particles, but can measure the radiation that results from the interaction of β-particles with atomic nuclei in the object, which is defined as bremsstrahlung (BS) radiation. Nevertheless, the ion chamber should be calibrated to measure 90Y, for which the calibration factor is calculated from a standard 90Y source.
The delivery vial contains the activity of 90Y-microspheres in 5 ml (SIR-Spheres™) or 0.6 ml (Theraspheres™) of water. Once the received activity has been measured, a simple ratio between the prescribed activity and the known activity per volume can be used to calculate the volume of solution to be drawn up.
Activity measurements should be conducted using fully suspended microspheres to avoid inconsistencies associated with self-shielding due to geometry changes.
3.2 Treatment Administration
The administration of 90Y-microspheres is performed through a catheter placed in the arterial hepatic vasculature, for which this procedure must be carried out in the interventional radiology catheterisation laboratory. Additionally, a delivery system that allows the administration to be carried out in a step by step manner is necessary to avoid an early full embolisation of vasculature that restricts the total infusion of the estimated dose. The administration equipment set (SIR-Sphere™ and TheraSphere™) consists of a methacrylate shield, the dose vial and inlet and outlet tubing with needles or a plunger assembly. A 5-ml syringe is used to infuse saline solution (Theraspheres™) or sterile water for injection (SIR-Spheres™) through the system. When the catheter is positioned at the treatment site and the nuclear physician verifies the integrity of the delivery system, the catheter is connected to the outlet tubing. Usually, slow and deliberate hand-injection of the 90Y-microspheres through the catheter system is adequate. Care should be taken not to allow too vigorous an injection rate, because this may result in leaks at points of potential weakness (e.g. septum, tubing connections). The infusion may be done with alternating injections of sterile water/saline solution and contrast medium, thereby allowing specific monitoring with the use of fluoroscopy to ensure that stasis is not reached. This approach also enables one to confirm that the flow of microspheres closely mimics that observed in the angiographic work-up. Flushing should be continued until optimal delivery of the microspheres is achieved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree