Mesothelioma
Todd M. Blodgett, MD
Janet Durick, MD
Sanjay Paidisetty, BS
Key Facts
Imaging Findings
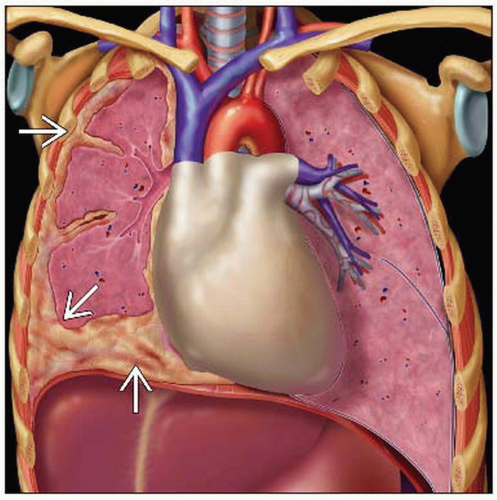
Pleural effusion &/or pleural thickening
80% of cases have primary arising from mesothelial surfaces of pleura
Also seen in peritoneum, pericardium, and tunica vaginalis
Distant metastases historically uncommon due to poor prognosis and rapid demise of patients
75% of pleural mesotheliomas are diffuse (usually malignant)
Nodular, irregular, unilateral pleural thickening
CT findings in mesothelioma by frequency
Pleural thickening
Thickening of interlobular fissures
Pleural effusion
Pleural calcification
Features helpful in distinguishing malignant from benign pleural disease include
Circumferential pleural thickening
Nodular pleural thickening
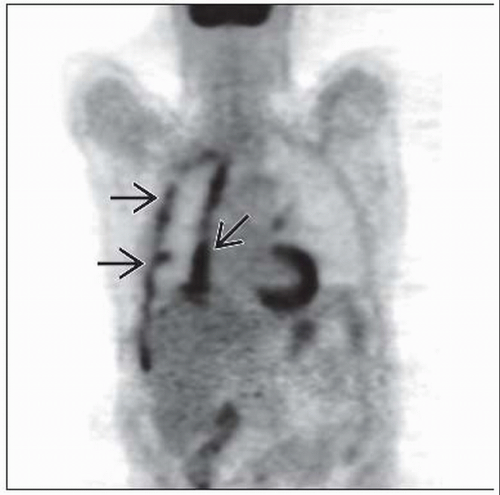
Mesothelioma generally FDG avid
FDG PET accurate for prediction of response with serial changes in tumor tracer uptake following one cycle of chemotherapy
Top Differential Diagnoses
Chronic Organized Empyema
Metastatic Tumor to Pleura, Especially Adenocarcinoma
Infection Processes (e.g., Actinomycosis, Tuberculosis, Nocardiosis)
Asbestos-related Pleural Disease
TERMINOLOGY
Abbreviations and Synonyms
Malignant mesothelioma (MM)
Peritoneal mesothelioma (PM)
Benign variant: Asbestos-related benign pleural disease
Definitions
Primary neoplasm arising from mesothelial cells that line body cavities
IMAGING FINDINGS
General Features
Best diagnostic clue
Chest: Pleural effusion, thickening, calcification
Abdomen: Omental caking or peritoneal masses
Location
60-80% of cases have primary arising from mesothelial surfaces of pleura
Also seen in peritoneum, pericardium, and tunica vaginalis
Compared with other intrathoracic malignancies, lymphatic pattern of spread of mesothelioma is unpredictable
Distant metastases historically uncommon due to poor prognosis and rapid demise of patients
Recent reports more common
Reported in brain, lung, bone, adrenals, peritoneum, abdominal lymph nodes, and abdominal wall
Size: Focal masses may grow to several centimeters
Morphology
75% of pleural mesotheliomas are diffuse desmoplastic type (usually malignant)
Diffuse disease may envelop abdominal viscera
Remainder are focal, producing large mass with scattered pleural/peritoneal nodules
Imaging Recommendations
Best imaging tool
Although not routinely used, PET/CECT may offer best information for diagnosis, staging, and response to therapy
CT sensitive but not specific for invasion
Protocol advice
Modified breathing algorithms to reduce misregistration along diaphragm
Coronal reformation of CECT may aid in detection of mesothelioma
Water or oral contrast to distend bowel loops
CT Findings
Pleural disease best imaged with contrast enhancement at 45-60 second delayed scan time
Extent of pleural and extrapleural involvement is well-evaluated on CT
Most common findings
Irregular, unilateral thickening of the pleura in a nodular, concentric, or plaque-like configuration
Pleural effusion
Pleural effusion commonly fills 1/3 to 2/3 of hemithorax
Pleural thickening may present with
Greater thickness at bases
Thickening of interlobular fissures
Calcification
Contraction of hemithorax in 40%
Chest wall invasion (difficult to detect based on irregularity alone)
Bilaterality in 20%
Findings of local invasion
Irregular contour along inferior aspect of diaphragm
Invasion of endothoracic fat
Loss of normal adjacent fat planes
Infiltration along biopsy tract or surgical incision seen in 20%
Greater than 50% circumferential encasement of mediastinal structure
Findings of extrathoracic spread
Soft tissue mass encasing hemidiaphragm
Absence of fat plane between diaphragm and abdominal organs
Liver metastases may rarely present with diffuse calcification
Findings typical of malignant vs. benign disease
Involvement of mediastinal pleura
Nodular pleural thickening
Greater than 1 cm thickening of parietal pleura
Circumferential pleural thickening
Response to therapy
Modified RECIST criteria evaluating thickness of involved pleura
Must decrease by 30% to indicate partial response to therapy
In one study, 47% of partial responders imaged with CT were detected after one cycle of chemotherapy
Nuclear Medicine Findings
Initial Diagnosis
Mesothelioma generally FDG avid
Mild or absent FDG uptake has been reported in some patients with mesothelioma of epithelial subtype
SUV of 2.0 used as cutoff for suspicion of malignancy in pleural lesions
FDG PET pattern and intensity do not allow differentiation of
Subtypes of malignant pleural mesothelioma
Mesothelioma from adenocarcinoma or sarcoma
Staging
PET/CT suited for
Detection of unsuspected nodal and occult distant metastases
Particularly useful for staging mediastinal nodal involvement
Sensitivity, specificity for T4 disease: 67% and 93%
PET alone sensitivity 19%
Limited for evaluation of nodal mets
38% sensitivity in one study
Insensitive for microscopic disease
False positives from inflammatory/infectious etiologies
e.g., talc pleurodesis, benign inflammatory pleuritis, parapneumonic effusion, tuberculous pleuritis
Limited for determining presence/extent of local tumor invasion
Response to therapy
FDG PET accurate for prediction of response following one cycle of chemotherapy
Defining tumor volume is laborious and error-prone due to “rind-like” morphology of mesothelioma
Tumor glycolysis volume (TGV) has been used effectively for accurate prognostic information
TGV superior to max SUV or CT response after one cycle of chemotherapy for predicting survival
Morphology
Tumors at earlier stage tend to have focal or linear patterns
Mixed and encasing patterns are indicative of more advanced disease
PET/CT and CT alone differ in TNM classification in up to 50% of patients
50% of discordances clinically relevant
PET/CT upstaged 12% of patients with noncurable disease and downstaged 12% of patients to curable disease
Prognosis
Best: Low SUV and epithelial histology; median survival 24 months
Worst: High SUV and sarcomatoid histology; median survival 14 months
High SUV tumors associated with 3.3x higher risk of death than low SUV tumors
Intensity of FDG uptake by primary shown to have poor correlation with histologic grade, good correlation with surgical stage
DIFFERENTIAL DIAGNOSIS
Asbestos Related Pleural Disease
Typically less FDG avid
Often indistinguishable from mesothelioma on CT
Look for absence of malignant findings
Continuous pleural “rind”
Pleural nodularity
Thickening > 1 cm
Involvement of mediastinal pleura
Benign disease may also demonstrate nodular pleural thickening
Biopsy recommended for equivocal cases
Calcified plaques are sign of asbestos exposure, not precursor to mesothelioma
Congestive Heart Failure
Interstitial and perivascular edema may develop, most prominent at lung bases
Large pleural effusions and alveolar edema in perihilar and lower lobe distribution
Correlate clinically with follow-up imaging
Chronic Organized Empyema
Gas bubbles in pleural fluid collection virtually diagnostic of empyema
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree