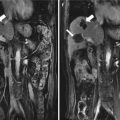
Fig. 28.1
(a) A real-time virtual navigation system. The transmitter is fixed to the operation bed (arrow). (b) A magnetic sensor is applied to the sonographic probe (arrow). (c) A magnetic sensor is mounted on the microwave (MW) antenna
There are three registration methods of a second modality images data to intraprocedural US images: internal markers method, external markers (the markers will cling to the surface of patients’ abdomen before CT or MRI examination) method, and topographical (xiphoid, sternum, and umbilicus) landmarks method. Among those, internal markers method is used mostly.
The accuracy of navigation system in biopsy and ablation procedures has been reported in a range that is consistent with clinical utility [17–19]. In the study by Penzkofer et al. [20], 23 patients underwent image-guided interventions using EM tracking navigation with a reported spatial accuracy of 3.1 ± 2.1 mm. Our previous ex vivo experimental results [21] showed the accuracy of the matched US-CT images was very satisfactory in the fact that it was found there was a mean discrepancy of 1.63 ± 1.06 mm. Therefore, the accuracy of navigation system could satisfy the condition of MW ablation.
28.2.1 Microwave Ablation Procedures Assisted by Navigation System
MW ablation procedures assisted by navigation system are introduced as follows based on the virtual navigation system (taking MyLab90 System equipped with Virtual Navigator, Esaote SpA, Genoa, Italy as example). Prior to MW ablation, DICOM volume data from MRI (or CT) were loaded on the fusion imaging system apparatus. The patient lay in the operation bed with supine position which is the same to the CT or MRI examination. B-mode ultrasound images and the second modality images (MRI or CT images) are synchronized using the internal markers at the best timing of the inspiration. First, a US image is selected as the first marker plane (sagittal part of portal vein, spleen vein, etc.) while the probe is perpendicular to the abdominal wall of the patient, and then the US image and the second modality image are adjusted in order to make the two displayed planes overlap completely. Second, another US image is selected as the second marker plane (right branch of portal vein, cysts, etc.), and the US image and the second modality image are made to overlap completely with the fine-tuning mode. Last, whether the fusion image is satisfied or not is verified, using the larger vessels of liver as a reference, when the patient was at the end of calm inspiration. If the distance of the same point between US image and the second modality image was less than 5 mm, the fusion image was considered successful; otherwise, we readjust the US image and the second modality image until the fusion image was satisfied. The time required for image fusion was recorded.
After the fusion image is completed, the MW ablation is performed under real-time virtual navigation system guidance with the patients under unconscious intravenous anesthesia (propofol and ketamine) in the operating room. A detailed protocol including the placement of the antennae, power output setting, emission time, and appropriate approach is worked out for each patient on an individual basis before treatment. Sterilizable biopsy kit (ABS 421, Esaote, Genoa, Italy) and sterilizable mounting brackets with disposable needle guide (DBS421/431, Protek Medical, Coralville IA, USA), with biopsy needle angles of 20° and 30°, have been used to guide the needle insertions. The tumors, well visible in the CT or MRI (and inconspicuous on US), have been “targeted” using the information coming from the fusion image. A 15G MW antenna (Kangyou Corporation, China) is inserted into the liver using real-time guidance and biopsy kit. The virtual needle is displayed computing its projection on the biopsy line. In general, MW ablation is performed at 50 W using one to two cooled-shaft antennae simultaneously. During MW ablation the hyperechoic area of ablation is monitored using gray-scale sonography, because the second modality image may be overlapped on the current ultrasound situation. The treatment session would be ended if the transient hyperechoic zone between antennae on gray-scale US merged and covered the target region. When withdrawing the antennae, the needle tracks are routinely cauterized to avoid tumor seeding and bleeding.
28.2.2 Clinical Use of Navigation System
The potential clinical indications for the use of navigation technique during microwave ablation are as follows: lesions seen only on CT or MR imaging and lesions not seen at all on US; composite ablations requiring multiple needle insertions, complex geometries, or difficult treatment plan; identification of safest pathway, given complex angle of insertion; and dome lesions or lesions under the ribs. Electromagnetic navigation is forbidden to use for the patient with a pacemaker, while optical navigation is available for this.
Navigation technique offers a distinct advantage in cases where the target lesion is not readily visible with conventional imaging guidance, such as ultrasound and CT, without iodinated contrast administration. Several studies have reported the usefulness of navigation technique for lesions that are indistinct, heterogeneous, or only visible on PET-CT [14, 22, 23]. Navigation technique may enhance ablation planning and execution, especially if US visualization is hindered by ablation gas. Ablation-planning software enables visualization of the potential ablation zone, depending on probe type, number, and position, to facilitate attempts for complete tumor coverage. Moreover, there may be advantages in terms of delivering a prescribed treatment plan or adding together multiple individual ablations to fully envelop a tumor with a composite ablation (i.e., multiple overlapping ablation volumes) [24–26].
From October 2009 to December 2012, 34 patients with 36 HCC nodules, consisting of 32 males and 2 female aged 42–78 years (mean, 59.1 ± 9.3 years), were performed microwave ablation assisted with navigation in our department. Of all patients, 33 patients had hepatitis B- or C-induced liver cirrhosis, and one patient had biliary cirrhosis (Child-Pugh A, 32; Child-Pugh B, 2). HCC was diagnosed using imaging analysis, including dynamic contrast-enhanced CT or MRI. The maximum diameter of the HCC nodules ranged from 9 to 38 mm (mean, 19.2 ± 7.3 mm). Among the 34 patients, five had received TACE within 1 month before MWA in this series.
We synchronized successfully B-mode ultrasound images with the second modality images (MRI or CT images) using the internal markers at the best timing of the inspiration for all the patients. The target HCC nodule and viable portion of the HCC could be detected with fusion image in all patients. The time required for image fusion was 5–30 min (mean, 12.2 ± 5.5 min). MWA was successfully performed in all patients. Of all 36 lesions, 35 lesions were successfully ablated according to the contrast-enhanced imaging 1 month after ablation. The technique effectiveness rate was 97.22 % (35/36). For the other one patient, the lesion was not ablated completely according to the contrast-enhanced MRI, and the ablated area was close to the lesion. Another MW ablation was performed for the patient. After MW ablation, mild pain and fever were noted, but no severe complications occurred. Even the tumor lesions directly beneath the diaphragm were ablated without side effects such as injury of the diaphragm. No thermal injuries to adjacent structures or organs occurred. The follow-up time was 6–24 months (median, 12 months) in our study. No local recurrence was observed in any patients (Figs. 28.2 and 28.3).







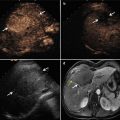
Fig. 28.2
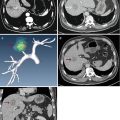
Images in a 54-year-old male with a 1.4 × 1.3 cm residual tumor of hepatocellular carcinoma (HCC) after transcatheter arterial chemoembolization in segment VII of the liver. (a) Contrast-enhanced magnetic resonance imaging (MRI) shows a residual tumor (arrows) adjacent to the diaphragm. (b) Conventional US could not detect the lesion. (c) Ultrasound (US) images and MR images were synchronized using the internal markers at the best timing of the inspiration. (d) MW antenna was inserted into the tumor under real-time virtual navigation system guidance. The virtual needle was displayed on the biopsy line (arrows). (e) Contrast-enhanced MRI obtained 1 month after MW ablation shows complete necrosis of the residual tumor (arrows)



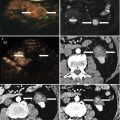
Fig. 28.3




Images in a 58-year-old male with a 2.0 × 1.5 cm lesion in segment VII of the liver. (a) Contrast-enhanced MRI shows a hyper-vascular tumor (arrows) in segment VII of the liver. (b) Conventional US could not detect the lesion. (c) US images and MR images were synchronized using the internal markers at the best timing of the inspiration. (d) MW antenna was inserted into the tumor under real-time virtual navigation system guidance. (e) Contrast-enhanced MRI obtained 1 month after MW ablation shows complete necrosis of the tumor (arrows)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree