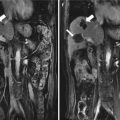
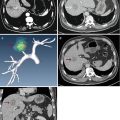
Fig. 14.1
The H&E staining slide of mice hepatocellular carcinoma (HCC) in situ after microwave ablation (MWA) (40×)
In situ tumor ablation (destruction) can involve tumor antigen release. Our in vitro experiment study has demonstrated that most of H22 cells underwent apoptosis (Fig. 14.2) and expressed HSP70 on the surface of cells after MWA of cell suspension with a temperature condition of 60 °C, but few cells expressed HSP70 before ablation (Fig. 14.3). It could be predicted that hyperthermia with temperature control of 60 °C might be immunogenic due to release of abundant levels of HSPs that accumulate during heating [28]. Hsp70 released during heating might contain tumor antigens and thus act like a molecular chaperone vaccine, by transporting tumor antigens to antigen-presenting cells and triggering activation of tumor-specific cytotoxic T lymphocyte (CTL) [29, 30]. In conventional surgical resection of colorectal liver metastases, it is known that values of carcinoembryonic antigen (CEA) fall rapidly, which reflects the elimination of the tumor load. While following RFA, patients show an initial rise and then a slow drop to background levels of CEA value, suggesting a slow release of immune reactive antigens from the tumor debris [31].



Fig. 14.2
The flow test result for H22 cells suspension after MWA with temperature control of 60 °C. Most cells have already undergone apoptosis in the upper right quadrant 2 h after MWA

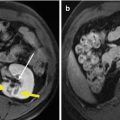
Fig. 14.3
The HSP70 expression on H22 cells before and after MWA by immunofluorescence (40×). (a) There are few H22 cells expressing HSP70 on the cytomembrane before MWA. (b) HSP70 expression both on cytomembrane and cytoplasm by most H22 cells 2 h after MWA
Systemically releasing antigens becomes available for professional antigen-presenting cells after ablation. As a kind of antigen-presenting cells, the DCs residing in the tumor-draining lymph node readily internalize antigens from the tumor microenvironment during the first 2 days after RFA [32]. And then the mature DCs can mediate antigen-specific cellular immunity via presentation of processed tumor antigens to T cells. Hence, the tumor antigen release after ablation could stimulate the tumor-specific immune response through the pinocytosis and presence of DCs.
14.2.2 Specific and Nonspecific Immune Response to Tumor After Ablation
The specific and nonspecific immune response belong to adaptive and innate immune response, respectively. The tumor-specific immunity often employs T lymphocyte to exclusively act against tumor cells, while the nonspecific immunity often employs NK cells and macrophagocyte to act against all pathogens including germ, virus, and tumor cells.
Zerbini et al. [33] showed increased IFN-γ production and cytotoxic activity of NK cells 4 weeks after RFA of HCC. By classifying the patients into high and low responders, these parameters gained predictive value on the efficacy of the ablative treatment. The result suggested the NK cells had the effect of inhibiting tumor development after RFA [34]. In patients with HCC and colorectal liver metastases, IFN-γ production (directed against autologous tumor tissue) was observed in both CD8+ and CD4+ T cells after RFA [33, 35] The CTLs possessed highly elevated cytotoxic activity as indicated by adenylate cyclase release [35]. Interestingly, cross-recognition between ablated and non-ablated tissue was observed, but responses to autologous non-tumor tissue were absent or weak [33]. These results suggest specificity for tumor antigens that are absent in normal tissue, thereby limiting autoimmune reactivity.
In cancer patients, only few studies have described the induction of specific immune responses after RFA. Napoletano et al. [36] reported that naïve and memory CD62L + T cells translocate to the tissues and that T cells produced IFN-γ in response, in cancer patients, to the tumor-associated MUC1 antigen, while humoral immune responses were unaffected by RFA treatment. The latter is in contrast to a study by Widenmeyer et al. [37] who concluded that tumor-associated antigen-specific antibodies increased within weeks to months after ablation in 6 out of 49 treated patients. Univariate analyses of parameters in 20 patients identified the number of tumor-associated antigen-specific CD8+ T cells as a significant prognostic factor for recurrence-free survival after RFA [13]. However, RFA induces weak immune responses that are only occasionally strong enough to lead to spontaneous regression [38]. Clinical data additionally shows that it does poorly protect against secondary growth, local recurrence, or intrahepatic metastasis and thus encompasses a high rate of intra-hepatic metastases and recurrent disease [39]. Similarly, Zerbini et al. [33] reported the absence of a correlation between enhancement of antitumor T cell responses and disease progression, which may be due to tumor escape mechanisms.
There have been few that reported about tumor-specific and nonspecific immune response following MWA of HCC mostly due to the tardy prevalence. However, MWA and RFA should have similar immune responses for HCC patients because of the similar mechanism and thermal efficiency between RFA and MWA.
Although the specific and nonspecific immune response following RFA of HCC have been reported, the high recurrence rate of HCC after ablation is still troublesome. It may be due to the weakness and transience of immune reaction. Hence, immunotherapy should be employed to overcome the hypoimmunity of HCC patients.
14.3 Efficacy of MWA Combined with Immunotherapy for HCC Management
14.3.1 Superantigen Administration After MWA
Cancer immunotherapy can be defined as a set of techniques aimed to eliminate malignant tumors through mechanisms involving immune system responses [40, 41].
Ablation provides a feasible strategy to administer immunomodulatory compounds in close proximity to the released antigens. Immunotherapy is currently one of the most studied novel cancer treatments. Therefore, combination therapies of ablation with other debulking strategies or immune stimulatory approaches are therefore of great interest.
In clinical, different strategies are employed to enhance the immune response to recurrence after MWA/RFA. Our group performed a clinical study to prospectively evaluate safety and effectiveness of injection of superantigen staphylococcal enterotoxin C (SEC) to peripheral area of ablated tumor on days 24 and 28, and 2, 5, and 7 months after MWA. The results displayed an extremely low concentration of SEC could induce strong nonspecific immune response by stimulation of T lymphocyte [42]. The overall survival rates for 1, 3, and 5 years were 93.3, 72.9, and 60.8 % in the SEC group and 94, 66, and 44.4 % in the control group. For patients with tumor of maximum diameter more than 30 mm, the disease-free survival rates at 1 and 3 years were 62 % versus 56.6 % and 37 % versus 9.4 % in the SEC and control group, respectively (p = 0.032). Adverse event related to SEC injection was associated with minimal systemic toxicity which included fever, general malaise, and muscular soreness. These results demonstrated that the local superantigen injection into ablated HCC early after MWA is safe and achieves longer overall survival as well as disease-free survival of larger tumors.
14.3.2 MWA or RFA Associated with Adoptive Immunotherapy to HCC
Immunotherapeutic strategies to overcome suppression and further stimulate DC and T cell activation are therefore of great interest. The application of these strategies as a monotherapy has so far resulted in meager responses in clinical trials. This may be because the patients enrolled did not respond to standard therapy and thus had a lower chance on clinical responses. Moreover, the stimulation of the immune system may particularly be of value in combination with standard therapies, like RFA, to reduce the tumor mass and inhibit the immunosuppressive tumor microenvironment. In fact, multiple studies were designed to evaluate the safety and effect of immunotherapy for patients with HCC after curative treatment including RFA or RFA combined with transcatheter arterial chemoembolization (TACE). Many results are inspiring. Cui et al. [43] have applied immune cells including NK cells, γδT cells, and cytokine-induced killer (CIK) cells to be infused intravenously to HCC patients for three or six courses after radical RFA. Compared to the patients in RFA alone group, the patients in combination strategy group get higher progression-free survival; furthermore, viral load of hepatitis C was decreased in two of three patients without antiviral therapy in RFA/CIT group, but was increased in RFA group. Huang et al. [44] have evaluated the clinical efficacy of autologous CIK cell transfusion in combination with TACE and RFA, compared to sequential therapy with TACE and RFA. The results showed that the patients in the TACE+RFA+CIK group had significantly longer overall survival (56 vs. 31 mo, p = 0.001) and progression-free survival (17 vs. 10 mo, p = 0.001) than those in the TACE+RFA group and no severe side effects occurred in the CIK cell transfusion patients, so they made a conclusion that the CIK cell immunotherapy may be a valuable therapeutic strategy to prevent recurrence and metastasis in HCC patients after TACE and RFA.
A meta-analysis of adjuvant therapy after potentially curative treatment for HCC has shown that adjuvant adoptive immunotherapy conferred significant benefit for RFS but not for overall survival, while adjuvant IFN therapy can improve both RFS and overall survival, but with accompanying severe side effects [45].
In addition to the application of singleness of combination of various immune cells like DC, CIK, and CTL and relative complexity operation was employed to enhance the immune response to reduce post-ablation recurrence of HCC. In our group, a phase I clinical study of combination therapy with MWA and cellular immunotherapy in HCC was carried out. Ten HCC (D ≤ 5 cm, fewer than three tumors) patients were treated with radical MWA and three courses of immunotherapy, which were started on the day of ablation, 2 weeks and 3 months after MWA. Immature DCs, CIK, and CTL were cultivated from peripheral blood of patients. Immature DCs were injected into the marginal area of ablated tumors under contrast-enhanced sonographic guidance 2 h after ablation. Tumor lysate-pulsed DC was injected into groin lymph nodes 11 and 100 days after ablation, while DC-CIK and CTL were injected into the abdominal cavity 5 days after ablation. CIK was infused intravenously 5 and 102 days after ablation. The infusion time of different immune cells was determined by the maximization of cell count and viability reflected in cell growth curve. The results displayed that no adverse effects of grade III/IV were observed and the viral load was decreased in 57.14 % (four of seven) of patients and was undetectable in two (28.6 %) patients without antiviral therapy. The percentage of CD4+CD25 high regulatory T lymphocytes decreased significantly and the percentage of CD8+CD28+ effector cells increased significantly 1 month after therapy. However, 6 months later, there was no significant difference [46].
In a following study, the immune response and clinical outcome of HCC patients attributed to immunotherapy after MWA were further investigated. The immunotherapy group includes 14 HCC patients who received 3–7 (mean 4.6) courses of immunotherapy after MWA, and the control group includes 42 HCC patients who have not received immunotherapy or other adjuvant therapy after radical MWA. All patients enrolled met the criteria of prior study. The results showed the absolute lymphocyte count, the proliferation rate of T lymphocyte, and the ratios of effector T lymphocyte subsets in immunotherapy group were significantly increased than those in the control group (p = 0.038, 0.011, 0.036). The values of albumin and cholinesterase in immunotherapy group were obviously increased after immunotherapy (p = 0.004, 0.037). The RFS and overall survival between two groups had no statistical difference possibly due to the limited sample in both groups and relatively short-term follow-up. However, the encouraging results in immune system responding to immunotherapy may help to against to HCC recurrence after MWA in a long run. Definite and positive results in disease-free survival rate and overall survival rate need further randomized controlled trial study with a long follow-up period.
The persistent confrontation between immune system and HCC cells lies on the whole process of occurrence, development, and deterioration of HCC. The dim clinical results of HCC mostly due to the high recurrence rate after radical treatment often imply the weakness of immune system against HCC. Multiple experimental and clinical studies involving immunotherapy for HCC patients following various treatments have given encouraging results in promoting tumor-specific immunity, directly killing tumor cells, or preventing post-treatment recurrence. However, the immunotherapy is far from perfect to applying in clinical, which even needs a further more abundant data to contribute to a mature strategy that HCC patients can benefit from.