Author
Patients
System
Tumor size (cm)
Follow-up (months)
CA (%)
LTP (%)
Five-year survival (%)
Major complication (%)
Seki et al. [7]
18
Microtaze
<2
11–33
100
0
N/A
0
Qian et al. [8]
22
FORSEA
2.1 ± 0.4
5.1
95.5
18.2
N/A
N/A
Liang et al. [9]
288
UMC-I
3.75 ± 1.58
31.41
NA
8
51
N/A
Lu et al. [10]
50
UMC-I
2.7 ± 1.5
18.1
94.4
≤2 cm:2
3-year, 73 %
0
>2 cm, 8
Kuang et al. [12]
74
FORSEA
0.8–8.0
17.4
93.2
5
N/A
12
Jiao et al. [13]
60
ECO-100A
1.0–8.0
17.17
92.71
5.21
N/A
0
Kawamoto et al. [15]
69
Microtaze OT-110M
≤4.0
54
93
28.6
63.9
NA
Itoh et al. [16]
60
Microtaze OT-110M
1.95
19
95
11.6
43.1
18.3
Liang et al. [17]
1,007
KY-2000
2.9 ± 1.8
17.3
97.1
5.9
59.8
2.2
Ikai et al. [18]
1,751
N/A
N/A
N/A
75.1
N/A
44.1
N/A
2.2 MWA Technique in HCC
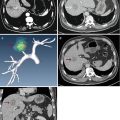
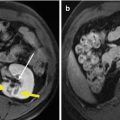
The general technique principles of MWA in HCC have been described in the previous chapter. According to the preliminary experimental study [11], in MWA of HCC, one antenna is inserted for tumors less than 2.0 cm and two for tumors measuring 2.0 cm or greater with an inter-antenna distance of no more than 2.5 cm. After one to three sessions of ablation, 1–3 days after the last course of a defined ablation protocol, contrast-enhanced imaging needs to be performed to evaluate the treatment efficacy. Technique effectiveness, namely, complete ablation, is defined as the absence of enhancement of any areas of the mass on a follow-up enhanced imaging performed 1 month after MWA, which represented the treatment that obtains initial success (Figs. 2.1 and 2.2). On the contrary, if residual unablated tumor exists, further ablation should be considered as soon as possible if the patient still met the criteria for MWA (Fig. 2.3).




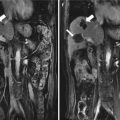
Fig. 2.1
Transverse images in a 64-year-old woman with two hepatocellular carcinoma (HCC) lesions treated by microwave ablation (MWA). (a) Contrast-enhanced magnetic resonance imaging (MRI) before ablation shows a 2.7 × 2.5 cm hyperintense nodule (long arrow) adjacent to intestinal tract (short arrow) at arterial phase. (b) Contrast-enhanced MRI before ablation shows a 3.3 × 2.9 cm hyperintense nodule (long arrow) adjacent to hilum (short arrow) at arterial phase. (c) Contrast-enhanced MRI scan obtained 1 month after ablation shows nonenhanced ablation zone (arrow) adjacent to intestinal tract. (d) Contrast-enhanced MRI scan obtained 1 month after ablation shows the nonenhanced ablation zone (arrow) adjacent to hilum

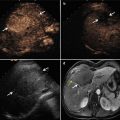
Fig. 2.2
Transverse images in a 41-year-old man with a single focus of HCC (2.5 × 2.0 cm) and accompanying cirrhosis. (a) Conventional ultrasound before ablation shows a hypoechoic nodule surrounded by several large vessels at liver hilum (arrows). (b) Contrast-enhanced ultrasound scan obtained before ablation shows a well-demarcated tumor (marks) with hyperenhancement at arterial phase. (c) Contrast-enhanced ultrasound scan obtained 24 months after ablation shows the nonenhanced ablation zone (marks)

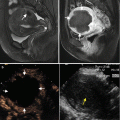
Fig. 2.3
Transverse images in a 54-year-old man with a single focus of HCC (3.7 × 3.6 cm) and accompanying cirrhosis. (a) Contrast-enhanced MRI before ablation shows a hyperintense nodule adjacent to the diaphragm at arterial phase (arrow). (b) Contrast-enhanced MRI scan obtained 1 month after ablation shows a 1.2 × 1.1 cm hyperintense nodule (short arrow) with hyperenhancement adjacent to ablation zone (long arrow) at arterial phase. (c) Contrast-enhanced MRI scan obtained 5 months after second ablation shows the nonenhanced ablation zone (arrow)
2.3 Results of Multicenter Studies for MWA of HCC
MWA of HCC was first adopted in Japan over two decades ago using a 2.45-GHz system (Microtaze; Heiwa Electronic Industrial Co., Ltd, Osaka, Japan) [7]; then this frequency has been in use in Europe and Asia since the 1990s. Microwave technology was first used in the USA in 2003 and involved a 915-MHz system (Vivant Medical, Inc., Mountain View, CA, USA) [23]. Now both 915-MHz and 2.45-GHz systems are employed in the clinical treatment of HCC worldwide. Despite its apparent advantages, MWA is still a relatively new ablative modality and current clinical trials mainly consist of single institution case series, albeit with promising results. Moreover, new MWA technology is rapidly becoming available for clinical use, and each new combination of generator and ablation antenna cannot be presumed to provide equivalent results. Therefore, it is increasingly important that clinical validation models are in place as technological innovation in this field progresses. Therefore, the multicenter study with prospective database is expected to provide a platform from which to critically evaluate a new modality. Currently, there are four such multi-institutional studies that have been reported. Lloyd et al. reported the preliminary results of international multicenter prospective study on microwave ablation of liver tumors [19]. One hundred and forty patients with 299 tumors from 18 international centers underwent MWA using a 2.45-GHz generator with a 5-mm antenna. Among all the 140 patients, 114 (81.4 %) were treated with MWA alone and 26 (18.6 %) were treated with MWA combined with resection. The median size of ablated lesions was 2.5 cm (range, 0.5–9.5 cm). Tumors were treated with a median of one application (range, 1–6 applications) for a median of 4 min (range, 0.5–30.0 min). A power setting of 100 W was used in 78.9 % of cases. They only showed that the results of the major morbidity were 8.3 % and in-hospital mortality was 1.9 %. Groeschl et al. reported a multi-institutional analysis of MWA for hepatic malignancies [24]. Four hundred and fifty patients with a total of 875 tumors (139 HCCs, 198 colorectal liver metastases, 61 neuroendocrine liver metastases, and 75 others) from 4 high-volume institutions were treated by MWA with 473 procedures. During a median follow-up of 18 months, complete ablation was confirmed for 97.0 % of tumors. A surgical approach (open, laparoscopic, or percutaneous) had no significant impact on complication rates and survival. The local recurrence rate was 6.0 % overall and was highest for HCC (10.1 %, P = 0.045) and percutaneously treated lesions (14.1 %, P = 0.014). In this large data set, tumor size of 3 cm or more predicted poorer recurrence-free survival (hazard ratio, 1.60, 95 %; CI, 1.02–2.50; P = 0.039), regardless of histology. Livraghi et al. reported a multicenter study focusing on the complications of MWA for liver tumors [25]. Seven hundred and thirty-six patients with 1.037 lesions (522 HCCs and 187 metastases) of 14 Italian centers were ablated by using a 2.45-GMHz generator delivering energy through a cooled miniature-choke MW antenna. Tumor size ranged from 0.5 to 10 cm. In 13 centers, the approach used was percutaneous, in 4 video laparoscopy and in 3 laparotomy. Results of this multicenter study confirmed no deaths occurred. Major complications occurred in 22 cases (2.9 %) and minor complications in 54 patients (7.3 %), which did not differ from those after RFA. The three multicenter studies all demonstrate the low morbidity rate of MWA for liver tumors, but they cannot provide the long-term survival and recurrence efficacy of MWA.
To assess the long-term efficacy of MWA for treatment-naive primary liver cancer with internally cooled probe and to examine additional factors that affect survival after MWA of liver cancer, between January 2005 and July 2010, a large database was generated by the recruitment and management of 1,007 patients with 1,363 lesions in seven Chinese centers with different levels of experience. The median follow-up period for all the patients after MWA was 17.3 months (range, 3–68.9 months). There were 819 men and 188 women with a mean age of 56.3 ± 11.1 years (range, 21–90 years). The lesion mean diameter was 2.9 ± 1.8 cm (range, 0.4–18.5 cm). Cancer types treated among patients with pathological diagnosis included 778 HCCs (96.4 %), 28 intrahepatic cholangiocarcinomas (3.5 %), and 1 cholangiohepatocellular carcinoma (0.1 %). Among the 1,363 lesions, 198 (14.5 %) are adjacent to gastrointestinal tract, 77 (5.6 %) to hepatic hilum, 364 (26.7 %) to the diaphragm or liver capsule, 167 (12.3 %) to large (≥3 mm) vessel, and 71 (5.2 %) to the gallbladder.
Nine hundred and seventy patients with 1,314 nodules underwent radical MWA treatment and 37 patients with 49 nodules received palliative treatment. The median ablation time was 450 S (range, 120–3,654 S) and the mean treatment session of patients was 1.2 ± 0.4 (range, 1–4). The results showed that technique effectiveness was achieved in 936 (96.5 %) patients and 1,276 (97.1 %) tumors for radical treatment, and LTP was observed in 75 (7.7 %) patients and 78 (5.9 %) tumors for radical treatment. HCC patients had 1-, 3-, and 5-year cumulative survival rates of 92.3, 73.1, and 60.8 %, respectively. The rate of major complication was 2.2 %. The multivariate analysis (Table 2.2, Fig. 2.2) showed that survival rates were related to sex, tumor number, tumor size, tumor pathology type, Child-Pugh classification, preablation alpha-fetoprotein level, liver cirrhosis, and presence of postablation extrahepatic metastasis. The hazard ratio of death was 2.67 times higher for ICC patients than that for HCC nodules, 1.59 times higher for patients with multiple nodules, and 2.39 times higher for patients with worse liver function (Fig. 2.4).
Table 2.2




Multivariate analysis of prognostic factors with Cox proportional hazards model
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree