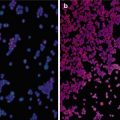
Fig. 4.1
Microwave ablation (MWA) in a 55-year-old woman with HCC on right lobe of the liver (S8), who refuses to undergo hepatic resection. (a) Preablation conventional ultrasound (US) scan shows a hypoechoic lesion with poor blood supply (arrows). (b) Contrast-enhanced US before MWA shows tumor enhancement in arterial phase with the size of 5.1 × 4.0 × 3.8 cm (arrows). (c) Contrast-enhanced US shows no enhancement of the ablation zone at 7 days after treatment. (d) Arterial phase magnetic resonance imaging (MRI) scan obtained 2 months after ablation shows hypoattenuating ablation zone (arrow) without enhancement

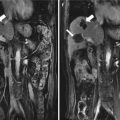
Fig. 4.2
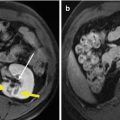
MWA in a 45-year-old woman with HCC on right lobe of the liver. (a) Preablation conventional ultrasound (US) scan shows a hypoechoic lesion with blood supply (arrows). (b) Contrast-enhanced US before MWA shows tumor enhancement in arterial phase with the size of 5.5 × 4.7 cm (arrows). (c) Conventional US shows two microwave antennas (mark) are placed in the tumor. (d) Contrast-enhanced US shows no enhancement of the ablation zone at 12 month after treatment (arrows). (e) MRI scan obtained 21 month after ablation shows hypoattenuating ablation zone (arrow) without enhancement
4.6 Therapeutic Efficacy, Assessment and Follow-Up
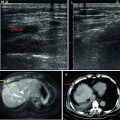
Every patient receives contrast-enhanced US 10–15 min after ablation to evaluate treatment response. Possible residual tumour is doubted if any abnormal nodular hypervascular region exists at the peripheral region of ablation. In addition, for large HCCs, the assessment of the possible residual tumour region between antennas is particularly critical. Complete ablation is considered as the entire tumour shows no enhancement on contrast-enhanced US. The follow-up period was calculated starting from the beginning of MWA. All patients were monitored with contrast-enhanced ultrasound, contrast-enhanced computed tomography or gadolinium-enhanced magnetic resonance imaging every 3–6 months (Fig. 4.3). A new lesion that appeared in or adjacent to the successfully treated nodule or an enlargement of the treated nodule is considered to be local recurrence. The presence of intrahepatic or extrahepatic new tumour nodules was defined as distant recurrence.


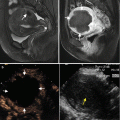
Fig. 4.3
MWA in a 75-year-old man with HCC that is adjacent to portal vein (yellow arrow) and gallbladder (blue arrow). (a) Preablation conventional US scan shows a hypoechoic lesion (arrows). (b) Contrast-enhanced US shows one hyperenhancement neoplasm with the size of 8.6 × 6.6 × 8.0 cm (arrows). (c) MRI scan obtained 25 months after ablation shows hypoattenuating ablation zone (white arrows) without enhancement
4.7 Clinical Efficacy of MWA in HCC (≥5 cm)
The research on MWA of large HCCs is few (Table 4.1). In 2001, Chen J. W. et al. [26] had proven that MWA could completely ablate 14 liver cancer nodules about 6 cm in diameter by suitably prolonging the irradiation duration and using rational multiple insertion schemes through ex vivo model. Kuang M. et al. [11] presented a complete ablation rate of 92 % (12 of 13 tumours) in large HCCs (5.1–8.0 cm), and Yu Z. et al. [17] reported a complete ablation rate of 100 % in four HCC lesions measuring >6 cm. Lately, Liu Y. et al. [8] treated 28 patients with HCC tumours measuring 5.1–8 cm using MWA and achieved a complete rate of 75 % and median survival month of 19. All these studies achieved favourable local effect. However, the samples are limited.
Table 4.1
Prognosis of patients with large-size HCC treated by MWA
Author | No. of patient | Diameter (cm) | CA (%) | LR (%) | DR (%) | Overall survival | ||
|---|---|---|---|---|---|---|---|---|
1- | 3- | 5-year | ||||||
Yin et al. [7] | 68 | ≥3.1 | 78.1 | 24.6 | 56.7 | 62.3 | 29.6 | 21.6 |
Liu et al. [8] | 80 | 3–8 | 87.5 | 22.2 | N/A | 81.1 | 56.5 | 34.6 |
Yin et al. [7] | 49 | N/A | 95.9 | 17.0 | N/A | N/A | N/A | N/A |
Kuang et al. [11] | 90 | 3–8 | 92.0 | 5.0 | N/A | N/A | N/A | N/A |
Chen et al. [27] | 99 | >4 | 94.0 | 18.7 | 15.2 | 89.9 | 53.1 | 28.6 |
Liu et al. [10] | 39 | >4 | 73.7–85.7 | 14.3–26.3 | N/A | N/A | N/A | N/A |
In our latest study, 107 patients with HCCs larger than 5 cm underwent percutaneous MWA with US guidance. Of these patients, 31 had initial HCC (mean diameter of 5.8 ± 0.9 cm) and 76 had recurrent HCC (mean diameter of 6.1 ± 1.4 cm). Ninety-one of 107 (85 %) patients achieved complete ablation (initial HCCs 90.3 %, recurrent HCCs 82.9 %). Incomplete ablation was gotten on the remaining 16 patients because the danger of tumours near important organs including inferior vena cava, port of liver, gallbladder, the ultrasound-guided blind regions, etc. The remains were treated by local injection of ethanol and/or comprehensive treatment. One to seven antenna insertions per patient were performed. The ablation session was no more than three per patient. The mean procedure time was 2,044 s. At a follow-up period ranged from 5 to 60 months, local recurrence was observed in 24 out of the 91 (26.4 %). In a univariate analysis, incomplete destruction of tumour was the risk factor of local recurrence. During the follow-up, distant recurrences developed in 57.9 % of patients after ablation, including new intrahepatic lesions in 45 patients, kidney metastases in 1 patient, lung metastases in 3 patients, retroperitoneal lymph metastases in 3 patient and multiple metastases in 11 patients. New intrahepatic tumours were observed in 13 of 31 (41.2 %) patients with initial HCC and in 49 of 76 (63.5 %) patients with recurrent HCC (p = 0.032). The 1-, 3- and 5-year overall survival rates were 80.3, 54.0 and 48.0 %, respectively, with a median survival of 23.8 ± 13.4 months. Of the initial HCCs, the 1, 3 and 5 overall survival rates were 87.0, 70.0 and 38.6 %, respectively. Of the recurrent HCCs, the probabilities of 1, 3 and 5 overall survival rates, respectively, were 77.6, 43.0 and 17.7 %. Univariate analysis indicated that patients with initial HCCs had better long-term survival (p = 0.001; Fig. 4.4). For patients with initial HCCs, the 1-, 3- and 5-year overall survival rates after the complete ablation were 87.0, 70.0 and 57 %, with mean survival of 31.7 ± 15.4 months. As the results for patients with recurrent HCCs were 77.6, 43.0 and 14.3 %, with mean survival 20.6 ± 11.1 months. Complete destruction of tumour was another significant prognostic factor that affected long-term survival (Fig. 4.5). Complete ablation had better long-term survival than incomplete ones (26.6 vs. 17.5 months, p = 0.033). Cox regression analysis confirmed that incomplete tumour ablation and recurrent tumours were independent unfavourable prognostic factors (p = 0.018).



Fig. 4.4
Univariate analysis indicated that patients with initial hepatocellular carcinoma (HCC) had better long-term survival

Fig. 4.5
Complete destruction of tumour was a prognostic factor that affected long-term survival
4.8 Complications
Side effects and minor complications of MWA for large HCC include slight to moderate postprocedural pain, a noninfective slight fever (≤37.5 °C), increase in liver transaminase levels (usually reverted to normal levels within 7 days), nausea, hydrothorax and skin burn [8, 10]. Liu Y. et al. reported a rate of 14.3 % (4/28) major complications in 5–8-cm HCC MWA [8]. In their study, one patient developed hepatic subcapsular hematoma, and acute renal dysfunction occurred in two patients. All were treated with conservative treatment. Long-time complications such as needle track tumour seeding had not been reported.
4.9 Other Local Techniques
4.9.1 TACE
According to current treatment guidelines, TACE has been established as the standard therapy for patients who are not eligible for curative therapies, especially for large, multiple and rich blood supply HCC [27, 28]. The survival benefit of TACE treatment has been proved in two randomised clinical trials [28, 29]. TACE can slow tumour progression and improve survival by combining the effect of targeted chemotherapy with ischemic necrosis by arterial embolization [31, 32]. It can also control or eliminate micro-metastasis, which cannot always be detected by ultrasound, CT or MRI. It was found to improve survival, with 1, 2 and 3 year overall survival rates of 82, 63 and 29 % [19]. However, the long-term outcome for patients with unresectable HCC treated with TACE was unsatisfactorily due to the inability to achieve complete tumour necrosis (<50 %) [20, 21, 30]. Repeated TACE was often needed to completely eradicate the residual tumours, but its efficiency was limited and the rate of tumour recurrence or relapse after initial remission or stable disease was very high. Additionally, repetitive TACE treatments might damage liver function reserve and decrease survival time. Studies have also proven that the effect of TACE was influenced by tumour size which decreases inactivation ability, especially for HCCs which are larger than 5 cm [33, 34]. Therefore, it is challenging and dissatisfactory for TACE alone to treat large HCC.
4.9.2 Radiofrequency Ablation (RFA)
Livraghi T. et al. [24] firstly evaluated the feasibility and effects of RFA in 46 tumours (5.1–9.1 cm). Complete necrosis was attained in 11 lesions (23.9 %), and nearly complete (90–99 %) necrosis was attained in 30 lesions (65.2 %). Medium tumours (3–5 cm) were treated successfully significantly more often than the large ones (5.1–9.1 cm). According to the study of Seror O.N. et al. [9], RFA performed on 28 HCC patients with a tumour dimension between 5 and 9 cm, the complete ablation rate was 81 % and the 2-year survival rate was 56 % in all patients. They reported that the complete ablation of tumours up to 8 cm was feasible and safe in patients with HCC and cirrhosis. Seror’s latest study used multiple sequential overlapping ablations to ensure adequate coverage and had indicated that multipolar RFA may overcome the main limitation of the percutaneous technique – namely, the size of the tumour. They showed that the complete ablation of tumours up to 8 cm was feasible and safe in patients with HCC and cirrhosis [10].
RFA has been a well-researched and widely used technique [18]. Compared with RFA, MWA uses high-frequency electromagnetic radiation, resulting primarily in active heating of surrounding tissues and more efficient energy deposition [11–14]. Our previous studies had showed that 915-MHz MW produced significantly larger ablation zone than all the RF ablations (P < 0.05) and 2,450-MHz MW ablation; meanwhile, the 2,450-MHz MW ablation zone was significantly larger than RF ablations (P < 0.05) in the same time. Owing to the above superiorities of MW, MW might be much suitable for large HCC ablation: (1) strong thermal efficiency would be achieved with same condition; (2) complete ablation could be attained in short time; and (3) fewer antenna insertions mean less invasions, less expenditure, easier technical procedure and, furthermore, lower complication rates.
4.9.3 Combined Therapy
Compared with small-/middle-sized HCC, the treatment of large HCC is challenging. Recently, comprehensive therapy showed more favourable clinical efficacy rather than TACE or thermal ablation alone (Table 4.2). Either TACE or thermal ablation has its own limitations. In particular, neither can result in the adequate control of medium or large HCC [27, 28, 34–36]. Blood flow will lead to heat loss which may reduce the effectiveness of ablation. One possible way to increase the ablation zone might be to reduce or eliminate the heat loss which is mediated by tissue perfusion [37]. Blood flow to HCC lesions can be substantially reduced by the arterial embolization effect of TACE. Moreover, TACE has a strong antitumour effect on HCC lesions. The synergy between TACE and thermal ablation has been well described [25, 31–34, 37–41]. Occlusion of hepatic arterial flow by embolization reduces the heat-sink effects of hepatic blood flow on thermal coagulation and increases the necrotic area induced by ablation [22]. In addition, the effect of anticancer agents on cancer cells may be enhanced by hyperthermia. Furthermore, iodised oil and gelatin sponge particles used in TACE fill the peripheral portal vein around the tumour by going through multiple arterioportal communications [38], thus reducing the portal venous flow and targeting undetected satellite lesions outside of the ablative area [23]. Moreover, ablation can disrupt intratumoral septa, which usually happens after TACE, and facilitates heat distribution within the tumour. At present, the combination of TACE with immediate synchronous RFA for unresectable and large HCC was recommended. Wang Z. J. et al. [42] reported that compared with conventional sequential therapy, immediate combination therapy could be fully synergistic: (1) lipiodol precipitation in the lesion wraps around and inactivates the surrounding tissue of the tumour, thereby preventing recurrence from residual tumours, and (2) lipiodol cannot be released and chemotherapeutics in lipiodol can inhibit tumours due to their high accumulative concentration.
Table 4.2




Minimally invasive treatment results of patients with large-size HCC
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree