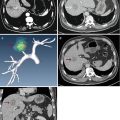
Fig. 1.1
Photographs of microwave equipment. (a) Intelligent microwave generator. (b) Prototype internally cooled microwave antenna with different shaft length (10–18 cm) and active tip (3–22 mm). The diameters of the applicators vary from 1.6 to 1.8 mm
Over the years, there have been continued efforts focusing on increasing the coagulation diameters by refinement of the antenna and generator. The first-generation system including Microtaze (Heiwa Denshi Kogyo, Osaka, Japan), UMC-I, and FORSEA system (both produced in China) with the needle antenna of 1.4–2.0 mm in diameter can create a coagulation zone of (3.7–5.8) × (2.6–2.8) cm in diameter when operated at 2,450 MHz. However, it is plagued by higher-power feedback; temperature of the antenna shaft rises quickly which can cause elongation of coagulation zone along the shaft due to thermal conduction and result in skin burn. Consequently, protective cooling of the skin is routinely used during ablation and the application of microwave emission is largely limited. Charring along the needle shaft may decrease energy deposited in the direction perpendicular to the shaft and reduce the short-axis diameter of coagulation. In order to keep off overheating of the shaft, to avoid skin injury, and to permit further deposition of energy into tissue with low impedance during ablation, cooled-shaft antennas have been developed in recent years. Inside the shaft lumen, there are dual channels through which chilled distilled water is circulated by a peristaltic pump continuously cooling the shaft. As shaft temperature can be effectively kept low, higher-power output and longer treatment duration are allowed which can deliver more energy into the tissue without causing skin burn. The cooled-shaft antenna has facilitated remarkable progress in obtaining larger ablation zone [27, 42].
With further improvement, currently, two kinds of frequencies—915 and 2,450 MHz—are used for MWA. The equipment with 915 MHz frequency is a newly developed instrument which can penetrate more deeply than that with 2,450 MHz and may yield larger ablation zone with the size of (5.2–5.8) × (3.0–3.8) cm [43]. Though MWA is mainly clinically used in eastern Asian countries, Western countries have attached great importance to it and begin to develop their own MWA systems [44]. And some other types of antennas such as loop-shaped antennas and triaxial antenna are also proposed but have not acquired wide use clinically [45, 46].
Some radiofrequency equipments contain a thermocouple in the nickel-titanium lateral tine of expandable electrode tip to allow temperature recording and monitoring during the ablation procedure, with the aim of ensuring that the maximum energy be applied by using the standard algorithm with the system [47]. Some MW machines are also equipped with a thermal monitoring system which can continuously measure temperature in real time during ablation. Thermal monitoring needle (Fig. 1.2) can be classified into thermocouple and thermistor type with the diameter of 0.7–0.9 mm (20–22 gauge), which is introduced into the liver parenchyma through a nonconducting needle trocar. Thermal monitoring needle is inserted into the target area to monitor temperature in real time during ablation under ultrasound (US) guidance. The aims of temperature monitoring include (1) therapeutic, the temperature monitoring needle is inserted about 5–10 mm away from the tumor margin. The complete tumor necrosis is considered achieved when the temperature remains at 54 °C for at least 3 min or reaches 60 °C; (2) protective, for high-risk localized tumors (less than 5 mm from the bile duct, gastrointestinal tract, gallbladder, pelvis, and so on), the real-time temperature of tumor margin is recorded to ensure that temperature does not reach damaging levels. The temperature cutoff of ablation is set at 54 °C in the patients without a history of prior laparotomy or 50 °C in the patients with laparotomy history. (We controlled the monitoring of temperature in patients with laparotomy history lower than those in patients without laparotomy history. That is because bowel peristalsis in patients without laparotomy history would help to avoid persistent heating of the same area. Adhesion may occur and decrease bowel peristalsis, thus increasing the risk of thermal injury of the bowel loop in patients with laparotomy history.) Then the emission of microwave is restarted after the temperature decreases to 45 °C and just so in cycles until the entire tumor is completely encompassed by hot bulb [37].


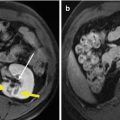
Fig. 1.2
Thermal monitoring needle with the size of 0.8 mm (21 G), which can be connected to the microwave equipment
1.3 Procedure
1.3.1 Indications (Taking Liver Cancer as Example)
Given the complexity of the hepatic malignancy, multidisciplinary assessment of tumor stage, liver function, and physical status is required for proper therapeutic planning. In general, the indications for MWA are broad. One important application is to treat patients who are not considered surgical candidates. Included in this category are patients with inadequate liver remnant to tolerate resection, tumor multinodularity, and unresectable lesions at difficult anatomical locations or patients who decline resection. Previous MWA was limited to treat small liver tumors, with the improvement of antenna and treatment strategy; lesions greater than 5 cm (5.0–8.0 cm) can also be effectively ablated [10, 39, 42].
For patients with early-stage primary liver cancer and limited metastases, MWA should be considered as curative therapy. The inclusion criteria are (1) a single nodule with a diameter smaller than 5 cm or a maximum of three nodules with a diameter smaller than 3 cm; (2) absence of portal vein cancerous thrombus; and (3) no extrahepatic spread to the surrounding lymph nodes, lungs, abdominal organs, or bone.
Palliative treatment criteria for MWA include patients (1) with lesion larger than 5 cm in diameter or multiple lesions, (2) suffering from a small extrahepatic tumor burden, and (3) unsuitable for other modalities and capable of tolerating the MWA procedure.
1.3.2 Contraindications (Taking Liver Cancer as Example)
Contraindications include patients who have (1) clinical evident liver function failure, such as massive ascites or hepatic encephalopathy or with a trancelike state; (2) severe blood coagulation dysfunction (prothrombin time >30 s, prothrombin activity <40 %, and platelet count <30 cells × 109/L); (3) high intrahepatic tumor burden (tumor volume >70 % of the target liver volume or multiple tumor nodules) or high extrahepatic tumor burden; (4) acute or active inflammatory lesions at any organ; (5) acute or severe chronic multiple organ dysfunction, including renal failure, pulmonary insufficiency, or heart dysfunction; and (6) relative contraindication that concerns medical risk for the tumor proximity to the diaphragm, gastrointestinal tract, gallbladder, pancreas, hepatic hilum, and major bile duct or vessels, which may require adjunctive techniques to prevent off-target heating of adjacent structures during the ablation procedure.
1.3.3 Patient Preparation and Data Required
Patients should be accurately evaluated through clinical history, physical examination, laboratory test, and performance status before MWA. Pre-therapy test of serum liver and renal function, respiratory and circulation function, cholinesterase, blood cell count, tumor markers, and coagulation should be known before the procedure. The impaired function needs to be corrected to withstand the ablation procedures. A full imaging work-up (a combination of contrast-enhanced imaging including US, computed tomography (CT), or magnetic resonance imaging (MRI)) should be performed to accurately stage and locate the lesions and exclude venous thrombosis and metastases before ablation.
Patients should receive both written and verbal information about the ablation procedure prior to therapy. Informed written consent must be obtained from each patient. Patients should be informed that MWA is not likely to cure their disease and is a palliative treatment directed to their liver lesions. Patients must be informed of the potential side effects of MWA as well.
1.3.4 Techniques
Similar to RFA, MWA can be performed percutaneously, laparoscopically, and thoracoscopically or at laparotomy as well. Whenever possible, MWA should be performed percutaneously for its least invasion, relatively low cost, and repeatability. General anesthesia with mechanical ventilation is required for laparoscopic or laparotomy approach. However, intravenous anesthesia combined with local anesthesia is usually sufficient for percutaneous approach. Detailed techniques have been described in the guideline of MWA in liver malignancy [37] and as follows: Patients are laid in the interventional US suite. US is performed to choose the safest needle access. Local anesthesia or plus intravenous conscious analgesia-sedation is usually sufficient for percutaneous MWA approach. After local anesthesia, the skin is pricked with a small lancet, and the antenna is placed into the chosen area of the tumor. In multiple needle procedure, two or three prefixed puncture lines are done. Two or three active needle antennas directly connected to the microwave generator are inserted into the tumor in parallel 1.0–2.5 cm apart. Thermal dosimetry of a single MWA applicator is dependent not only on tissue type but also on the amount of energy delivered to the tissue and the distance of the critical ablation margin from the applicator. For the patients’ breathing, cooperation to complete the insertion is needed; intravenous conscious analgesia-sedation is induced associated with standard hemodynamic monitoring after placing all the antennas. At each insertion, the tip of the needle is placed in the deepest part of the tumor. Multiple thermal lesions are produced along the needle antenna’s major axis by simply withdrawing the needle from the preceding thermal lesion and reactivating the microwave generator. If necessary, based on tumor size, multiple overlapping ablations are usually needed to envelope the entire tumor with a safety margin. Generally, the microwave energy is set at 50–80 W for 5–10 min in a session.
Size of the ablation zone can be roughly judged by an expanding hyperechoic area during the procedure. To have accurate assessment of the treatment efficacy, the thermal monitoring system attached to the microwave system can be used during MWA. One to three thermal monitor needles are placed at different sites 5–10 mm outside the tumor. The thermal monitor needle can be introduced into the parenchyma through 18 gauge, 70 mm length, nonconducting needle trocars (Hakko Co., Ltd, Japan). If the measured temperature does not reach 60 °C by the end of treatment or not remain at 54 °C for at least 3 min, the ablation is prolonged until the desired temperature is reached. When withdrawing the antenna, the needle track needs to be coagulated with the circulated distilled water in the shaft channel stopped to prevent bleeding and tumor cell seeding.
This ablation therapy includes a 5–10 mm ablative margin of apparently healthy tissue adjacent to the lesion to avoid local tumor progression for microscopic foci of disease and the uncertainty that often exists regarding the precise location of actual tumor margins. For patients with severe liver cirrhosis or the lesion adjacent to critical organ, an ablation margin of 5 mm or conformal ablation fitting tumor shape and contour is recommended to ensure a safe and radical treatment, and otherwise, a 10 mm enough margin is preferred. Reducing the tumor bulk is the strategy for patients who underwent palliative ablation treatment.
1.3.5 Care After MWA
After the MWA procedure, the punctured site is covered with a sterile dressing under pressure. The patient then needs to undergo a recovery for 4–6 h of bed rest. If necessary, the patients are observed for two to three additional days and discharged from the hospital when they feel no severe pain or when their body temperature does not exceed 38 °C.
1.3.6 Therapeutic Efficacy Assessment and Follow-Up
In recent years, contrast-enhanced US has been employed for immediate assessment of technical success which can be performed 10–15 min after MWA [48]. If the foci of nodular enhancement in or around the treated tumor are observed, a next MWA session with an identical device is performed as part of another course of treatment. Contrast-enhanced imaging needs to be performed at 1 month after the last course of a defined ablation protocol. If irregular peripheral enhancement occurred, which represents residual unablated tumor, this sign indicates incomplete ablation, and further treatment should be considered as soon as possible if the patient still meets the criteria for MWA. On the contrary, if complete ablation is achieved, then routine contrast-enhanced US, CT, or MRI and serum tumor marker are repeated at 3 months after MWA and then at 6-month intervals.
1.4 Clinical Applications
Though RFA remains the most widely used thermoablative technique worldwide, MWA as another effective local thermal ablation technique has undergone tremendous progress due to technical advances. Initially MWA was limited to treat small liver cancer, with the improvement of antenna and treatment strategy; large liver cancer greater than 5 cm can also be effectively ablated [49, 50]. In addition, MWA has expanded its clinical application field to multiple solid tumors including the kidney, adrenal, spleen, thyroid, lung, abdominal wall, and uterus [5, 8, 13, 17, 19, 21, 51].
The therapeutic efficacy of MWA can be augmented by other therapies. Similar to other thermal ablation techniques, the coagulation diameters for MWA are also influenced by perfusion-mediated cooling. Interruption of hepatic blood flow can significantly increase the coagulation diameters [52, 53]. Transcatheter arterial chemoembolization (TACE) is an effective method for reducing the blood flow of tumors and controlling the large tumors because of blocking artery effect. MWA combined with TACE may yield increased ablation volume and can destroy the peripheral part of the tumor remaining viable after TACE, whereas TACE may possibly control microscopic intrahepatic metastasis that cannot be treated by MWA [54]. Combination therapy with MWA and percutaneous ethanol injection can also increase the treatment efficacy, especially for tumors adjacent to vital organs [55]. For patients with high-risk localized tumors (tumor adjacent to important organs and tissues including the diaphragm, gastrointestinal tract, hilum, and major bile duct or vessels), combination of additional multiple techniques (artificial ascites, artificial pleural effusion, intraductal saline perfusion, and radioactive particle implantation) with MWA can also ensure favorable effect and low complications [56–58], which make MWA in the treatment of dangerous site tumors become feasible without sacrificing the therapeutic efficacy.
US as guidance tool has several limitations including the occasional poor lesion visualization as a result of a lack of innate tissue conspicuity or overlying bone- or gas-containing structures. MWA assisted by a real-time virtual navigation system is a feasible and efficient treatment of patients with lesions undetectable by conventional US [59].
In general, the indications for MWA are broad. One important application is to treat patients who are not considered surgical candidates. Included in this category are patients with unresectable tumor, patients with tumor at difficult anatomical locations, and patients who are too severely debilitated to tolerate resection. Similar to indications for RFA, MWA is also applicable to achieve curative therapy for small and early-stage liver or renal cancers with minimal invasion. MWA has achieved similar effect compared with surgery, RFA, and percutaneous ethanol injection treatment for hepatocellular carcinoma [60–62]. Long-term survival data and large-scale prospectively randomized controlled trials comparing it with other modalities, especially with RFA for ultimately determining its effectiveness, are earnestly anticipated.
1.5 Conclusions
MWA is a promising minimally invasive technique with many thermal characteristic advantages for the treatment of solid tumors. It can be performed safely using percutaneous, laparoscopic, or open surgical techniques. Advances in antenna design, treatment strategy, and combined therapies are anticipated to improve the therapeutic outcome of MWA in the future, making it a clinically important treatment option.