Fig. 37.1
The Fluostick™, optical head and control box
We have seen previously that, in order to reach an accurate fluorescence performance, the whole optical acquisition chain must be optimized for NIR light. Filtering is a critical part of the system. The filters used to collect the fluorescence signal are custom-made and particularly optimized for ICG. Behind the emission filters, the optical lens of the system is NIR coated to avoid reflection and to collect the maximum of incoming signal. A compromise is found between the F-number of the lens and the focal length to preserve the depth of field of our imaging system. It is particularly important considering the fact that the system is handheld and not sealed to a mechanical arm. The CCD sensor selected for the camera is NIR optimized. With a 1/3″ in. size and a definition of 752 × 576 px, it offers a generous pixel size of 6.5 μm and a high well capacity directly linked to a good dynamic range. The excitation is provided by a laser located in the electrical box. An optical fiber drives it to the head of the system. At the tip of the system, the fiber is expanded by an optical combination and spread quite uniformly and with no speckle to the imaged field. The optimized optical combination makes of Fluostick™ a class 1 laser system. At a 10 cm working distance, the laser illumination is 12.5 mW/cm² at 750 nm. At a 5 cm working distance, the laser illumination goes up to 25 mW/cm² at 750 nm, which is very comfortable for real-time and fast ICG visualization. In most of the cases, a FIGS system does not authorize the surgeon to keep the surgical shadowless light on, which could contain NIR light, during NIR acquisitions. The white light accessory of the Fluostick™ is able to provide a high-quality white light with a 4,000 lux power at 10 cm working distance and a very high color rendering index of 93. The aim is to be able to display a wide range of values of ICG concentrations on a single exposition. The analog signal, out of the optical head, is digitalized in the control box by high-range electronics before being sent to the computer. At the end, the system is able to image properly a quantity of ICG down to 5 pmol of product in solution with a very low background. Figure 37.2 shows the results of imaging three different 10 μL drops of ICG.


Fig. 37.2
Images of three different quantities of ICG, from left to right, 5, 10, and 100 pmol
The concentrations of the product are 0.5, 1, and 10 μM which correspond to 5, 10, and 100 pmol of ICG, respectively. Thanks to a very low background, the values of signal-to-noise ratio (SNR) are high (see Fig. 37.3). The spatial resolution of the image is around 70 μm. Figure 37.4 shows an image of an USAF1951 resolution chart acquired with the Fluostick™. In this picture, to the well-displayed group 2, element 6 corresponds to a resolution of 70.1 μm.
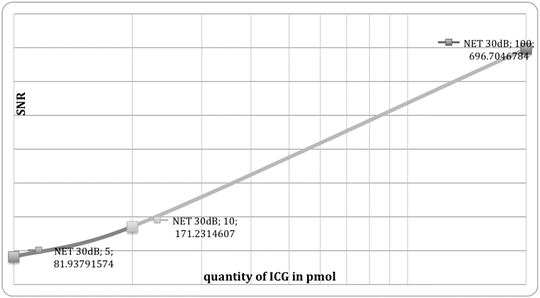
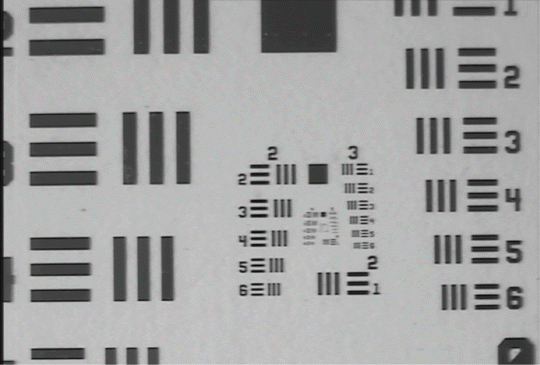

Fig. 37.3
Signal-to-noise ratio for different quantities of ICG

Fig. 37.4
Image of an USAF1951 resolution chart acquired with the Fluostick™
We showed previously that the Fluostick™ has good fluorescence imaging performances. The purpose of this study is to generate the smallest footprint possible with the best fluorescence performance. The initial specification was to develop a handheld instrument. The size constraints were to build the system in a footprint not bigger than 800 mm² with a length of 150 mm. Because the Fluostick™ is considered a handheld system, its frame rate should not be reasonably less than 25 frames per second. We can conclude that the system will collect the fluorescence signal with a 20 ms maximum exposition time. The three main technical choices made to reduce the size of the optical head were a fiber optical deported laser excitation; a single but powerful and high-quality LED for white light excitation; and a 1/3″ in. CCD analog sensor with the minimum of embedded electronics to interface it.
After investigation, the oblong format front section has been chosen for being the best compromise to fit the sensor, the laser excitation, and the white light illumination together. The oblong section fits a rectangle of 34 × 24 mm. Figure 37.5 displays the inside of the optical head and the disposition of the components. A curved shape has been given to the optical head with the purpose to be easier to handle. Moreover, the profiles of the system have been thought to fit the hand. A triangle form is designed to help the prehension of the optical head. Some cross sections of a 3D model of the optical head are shown in Fig. 37.6. The system is no longer than 140 mm.
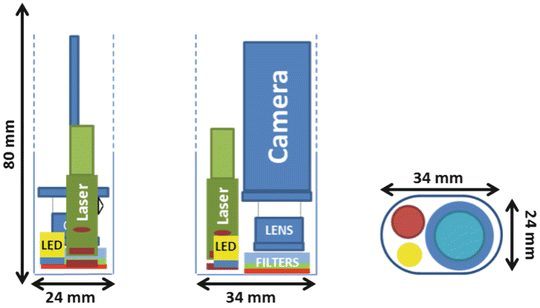


Fig. 37.5
Schematics, disposition of the components

Fig. 37.6
Cross sections of the Fluostick™ optical head
The entire design is thought to make the surgeon able to grip the system and acquire images in every situation. Table 37.1 presents several parameters of the Fluostick™ system.
Table 37.1
Fluostick™ review of performance
Footprint | 34 × 24 mm |
Length | 140 mm |
Weight | 125 g |
Working distance | 50–100 mm |
Field of view | 40 × 30 mm at 100 mm working distance |
Depth of field | 15 mm |
ICG sensitivity | 0.5 pmol |
Fluorescence excitation | 2.5 mW/cm2 at 50 mm working distance |
F-number | 1.8 |
Optical resolution | 70 μm |
Sensor definition | 756 × 576 px |
Sensor characteristics | High NIR sensitivity monochrome CCD, 25 fps |
White light intensity | 4,000/ux |
White light temperature | 4,000 K |
White color rendering index | 93 |
Dynamic range | 8 bits |
The Fluostick™, Improves Head and Neck Cancer Resection in a Preclinical Orthotopic Model
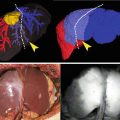
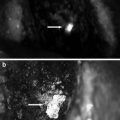
We used the Fluostick™ to guide head and neck squamous cell carcinoma (HNSCC) resection in an optimized orthotopic animal model for head and neck cancer. We performed a systemic administration of Angiostamp™ 800, an RGD-based probe that targets αvβ3 integrin, in nude mice presenting orthotopic HNSCC tumors developed after intraoral implantation of tumor fragments obtained from subcutaneous tumors derived from a human HNSCC. These tumors had a positive expression of αvβ3 integrin. Tumor resection was performed with and without the aid of Fluostick™. NIR optical imaging-guided surgery using Fluostick™ helped to detect fluorescent cancer residues that could remain unidentified if resection was done exclusively under visual guidance (Fig. 37.7). These residues were measured and analyzed microscopically and we found that Fluostick™ could detect fluorescent cancer foci as small as 185 μm. The detection of these residues had a positive impact on the recurrence-free survival rate of mice in comparison with mice which underwent tumor resection without the aid of Fluostick™. This preclinical stage is an important step before testing Fluostick™ in HNSCC resection in humans.
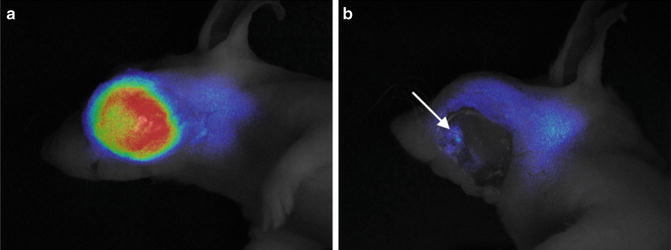

Fig. 37.7
Fluostick™-guided resection of HNSCC after systemic administration of Angiostamp™ 800. (a) Fluorescence of HNSCC orthotopic tumors. (b) Fluorescent residues (arrow) unintentionally left behind after total macroscopic resection of the tumor
Colorectal Peritoneal Carcinomatosis
The data from the French registry (FRANCIM network) show that the incidence of colorectal cancer (CRC) has been increasing during the last 30 years. Unfortunately, more than half of patients diagnosed with colorectal cancers present at diagnosis, or will present during their monitoring, visceral metastases (mainly liver) and peritoneal carcinomatosis (PC). Like isolated liver metastases, peritoneal carcinomatosis can be considered as a “regional stage” of metastatic disease. PC secondary to colorectal cancer is characterized by the development of solid tumor deposits on the peritoneal surface. Tumor implantation and growth may lead to invasion of any organ or structure covered by the peritoneum. This peritoneal dissemination can appear in 30–40 % of patients [21, 22]. The natural history of the disease is always fatal, with a median survival of 6 months [23]. However, since the early 1990s, several teams have conducted phase I–II studies to assess the interest of the combination of intraperitoneal chemotherapy with hyperthermia to cytoreductive surgery in the therapeutic management of this pathology. Only complete surgical cytoreduction associated with chemotherapy plus or minus intraperitoneal hyperthermia immediately provides a 5-year survival of 30–48 % [24–26]. The diagnosis of the initial tumor mass is an important prognostic factor of the disease itself. Curability is more easily obtained for limited carcinomatosis, carefully extirpated by the surgeon with a wide peritumoral tissue rather than bulky carcinomatosis which requires complex resections. The classical concept of R0 resection is no longer appropriate in the context of peritoneal surgery and was replaced by CC-0 (macroscopically complete cytoreduction) [27]. In addition, the tumor volume is closely related to potential metastatic disease which determines the progression-free survival. The “Peritoneal Cancer Index” (PCI) of Sugarbaker is a tool for precise quantitative assessment for carcinomatosis of gastrointestinal tumor origin. It is based on the distribution of tumor nodules and their dimension [27–29]. The peritoneal cavity is divided into 9 regions and 4 dials for the hail, 13 regions in total (Fig. 37.8).