, Gila Perk2, Natesa G. Pandian3, Hans-Joachim Nesser4 and Itzhak Kronzon2
(1)
Department of Cardiology, Cardiocentro Ticino, Lugano, Switzerland
(2)
Non-Invasive Cardiology, Lenox Hill Hospital, New York, NY, USA
(3)
Tufts University School of Medicine, Boston, MA, USA
(4)
Department of Medicine Elisabethinen, Teaching Hospital, Linz, Austria
Abstract
This chapter will discuss unusual transcutaneous, catheter-based procedures namely: transcutaneous closure of left ventricular pseudoaneurysm, pseudoaneurysm of intervalvular fibrosa of the aortic root, transcutaneous suction of right heart clot and valve in valve implant. In these procedures the relevant role of 3D TEE is described.
10.1 Transcatheter Closure of Left Ventricular Pseudoaneurysm
Left ventricular (LV) free wall rupture frequently leads to intrapericardial bleeding that rapidly results in cardiac tamponade and death. On rare occasions, the rupture is contained by pericardial and fibrous tissue, creating an LV pseudoaneurysm (PA). The pseudoaneurysm wall consists of pericardium alone, without any myocardial layers. Thus, this wall is thin and may easily rupture, causing bleeding into the chest cavity and death. Most pseudoaneurysms occur after myocardial infarction. They are more common in men and involve the posterior and lateral walls more commonly than the anterior wall. Pseudoaneurysms can also occur after mitral valve replacement, with a rupture near the posterior aspect of the mitral ring. Other conditions that may lead to LV pseudoaneurysm formation include aortic valve replacement, endocarditis with abscess formation, and cardiac trauma.
Echocardiography suggests or establishes the diagnosis in most cases. It shows a loculated echo-free space that communicates with the LV via a narrow neck, or tract. Doppler echocardiography demonstrates a characteristic to-and-fro flow in the communicating tract (from the LV to the pseudoaneurysm in systole and from the pseudoaneurysm to the ventricle in diastole).
Surgery is considered the treatment of choice. However, it is associated with high morbidity and mortality. In many patients with recent infarction, the tissue around the rupture is necrotic and friable, and the repair sutures may fall apart.
Transcatheter repair of pseudoaneurysm is now another option. It is best done under fluoroscopic as well as two-dimensional (2D) and real-time three-dimensional (3D) transesophageal echocardiography (TEE) and transthoracic echocardiography (TTE) surveillance and guidance.
Figure 10.1 shows a case of an anterior wall infarct with a large pseudoaneurysm. Figure 10.2 demonstrates the characteristic to-and-fro flow in the narrow communicating tract. After establishing the diagnosis, a decision was made to close the PA via an apical approach. Using 3D real-time TEE, a direct percutaneous needle puncture of the pseudoaneurysm was performed, and a wire and then a catheter were advanced from the puncture site through the communicating tract into the LV cavity (Fig. 10.3a). A closure device (Amplatzer; Golden Valley, Minnesota, USA) was then deployed to close the communication (Fig. 10.3b, c). Upon withdrawal of all catheters, another Amplatzer closure device was deployed at the pseudoaneurysm puncture site. The patient recovered without complications.
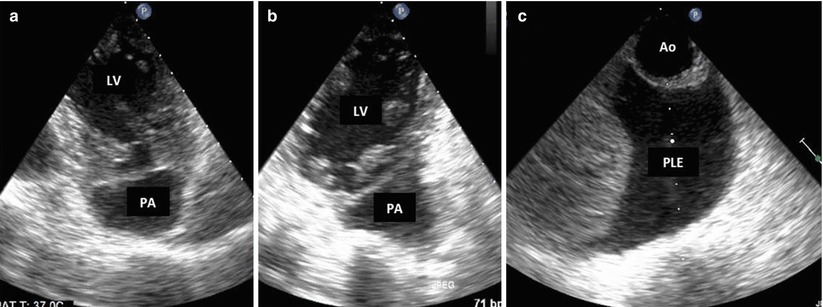
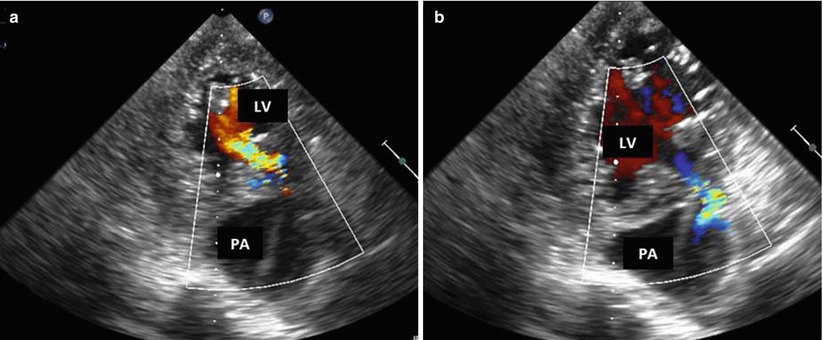
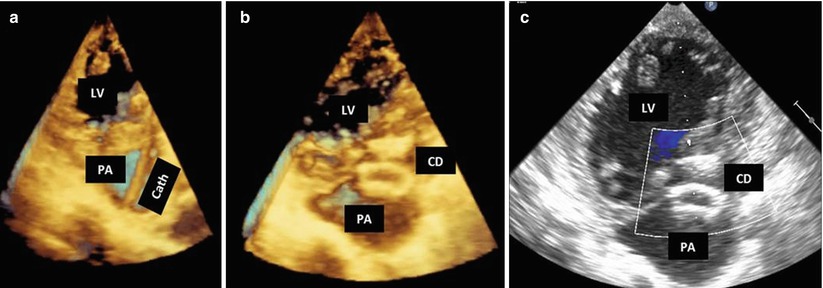

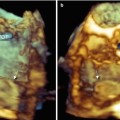
Fig. 10.1
Left ventricular (LV) pseudoaneurysm (PA). (a, b) 2D TEE, deep transgastric view after anteroseptal myocardial infarction, this patient developed a loculated echo-free space suggestive of PA. (c) New left pleural effusion (PLE) is noted in front of the aorta (Ao) and is suggestive of intrapleural bleeding

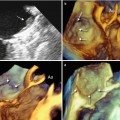
Fig. 10.2
Color Doppler interrogation of the left ventricular (LV) pseudoaneurysm (PA). (a) Diastolic frame. Note flow in the communicating tract from the PA into the LV. (b) Systolic frame, with blood flow from the LV into the PA

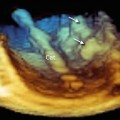
Fig. 10.3
Transcatheter closure of left ventricular (LV) pseudoaneurysm (PA). (a) A catheter (Cath) seen entering from the chest wall into the PA cavity and across the communication tract into the LV. (b) Closure device (CD) in place sealing the PA communication. (c) Color Doppler shows no residual flow between the PA and the LV
Figure 10.4 demonstrates an unusual pseudoaneurysm in a young patient who underwent aortic valve replacement, which was complicated by endocarditis. After clinical recovery, echocardiography revealed a pseudoaneurysm formation at the intervalvular fibrosa with a narrow communication between the LV outflow tract and the pseudoaneurysm (Fig. 10.4a). Characteristic to-and-fro flow was demonstrated by Doppler (Fig. 10.4b–d). After transcutaneous needle puncture of the LV apex, a wire and then a catheter were advanced, under 3D TEE guidance into the communication site (Fig. 10.5a, b). A coil was used to eliminate the pseudoaneurysm cavity (Fig. 10.5c). The catheter was then retrieved (Fig. 10.5d). An apical closure device (Amplatzer PDA) occluded the apical myocardial puncture site (Fig. 10.6




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree