This article is a summary of the most up-to-date applications of radiopharmaceuticals to the diagnosis and therapy of benign and malignant diseases involving endocrine or neuroendocrine organs of the head and neck, focusing on radiotracers approved by the US Food and Drug Administration, such as I-123- and I-131-sodium iodide, F-18-fluorodeoxyglucose, Tc99m-sestamibi, as well as the more recently approved tracers Ga-68 DOTATATE and Lu-177 DOTATATE.
Key points
- •
I-131 sodium iodide continues to be invaluable for remnant ablation and adjuvant therapy of well-differentiated thyroid cancer.
- •
Recent studies of patients after I-131 radioablative therapy have shown a small increased rate of solid cancer mortality and development of secondary cancers; further studies are needed.
- •
Tc99m-sestamibi remains a workhorse for workup of hyperparathyroidism, with F-18 fluorocholine PET/computed tomography scans showing promise in detection of parathyroid adenomas and multigland disease.
- •
Ga-68 DOTATATE is highly sensitive and specific for staging of somatostatin receptor-positive neuroendocrine tumors of the head and neck, having supplanted In-111 Octreoscan and playing a complementary role to I-123 meta-iodo-benzyl-guanidine.
Introduction
From the first production of radioactive iodine in 1936 by Enrico Fermi to the subsequent application of 130 I-sodium iodide (later 131 I) to treat hyperthyroid patients in 1946 by Drs Saul Hertz and others, molecular imaging and therapy of the head and neck was born.
Thyroid-benign disease
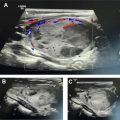
The thyroid gland descends during development from the foramen cecum at the base of the tongue to its location anterior to the trachea and inferior to the thyroid cartilage, weighs approximately 25 g, and measures 4 to 5 cm tall and 1 to 2 cm wide. Ectopic thyroid can occur anywhere along this descent, including at the base of tongue (lingual thyroid), hyoid bone (thyroglossal duct cyst), superior to either lobe or isthmus (pyramidal lobe), or in the mediastinum (substernal goiter). Symptoms of the hyperthyroid patient can be due to Graves disease (caused by autoantibodies to the thyroid-stimulating hormone [TSH] receptor), toxic multinodular goiter (caused by hyperplasia of follicular cells which become autonomous of TSH, and TSH receptor mutations), toxic adenoma, or thyroiditis. Unusual causes of thyrotoxicosis, including struma ovarii, lithium, and the Jod–Basdow effect (from iodinated contrast), and of hypothyroidism (lingual thyroid) have been previously reviewed. Nuclear techniques can differentiate between these entities and provide definitive treatment.
Imaging of the thyroid is performed with a thyroid uptake and scan, either with I-123 sodium iodide, or I-131 sodium iodide for the uptake and Tc99m-pertechnetate for the scan ( Table 1 ). On a thyroid scan in a patient with Graves’ disease, the thyroid gland seems to be homogeneous with convex contours, with a 24-hour iodine uptake of typically 60% to 80%. A mildly elevated or high-normal 24-hour uptake can sometimes be observed with rapid turnover Graves’ disease, when a 4-hour uptake (typically only obtained with I-123) would have been elevated. Although patients are often first treated medically with methimazole, radioablative therapy is ultimately pursued owing to the unfavorable side effect profile of thionamides. Radioablation for Graves’ disease with 131 I-sodium iodide typically uses a calculated method, with the dose proportional to the weight of the gland (in grams) and a constant (100–200 μCi/g), and inversely related to the uptake fraction; typically 10–15 mCi is the resulting dose. This method is selected to avoid radiation thyroiditis and ensure ablation success, but studies have shown that the risk of thyroid storm is very low and does not correlate with untreated hyperthyroidism before radioiodine ablation ; an empiric, fixed-dose method is similarly efficacious. Side effects include sialadenitis with possible xerostomia, sore throat, neck discomfort, and dysgeusia. Graves’ ophthalmopathy can be worsened by I-131 therapy, which is thought to result from deposition of antigen–antibody complexes. Compared with methimazole, radioiodine was associated with a lower recurrence but a higher incidence of ophthalmopathy in a large meta-analysis. ,
| Imaging Protocol | Notes |
| Thyroid uptake and scan – pediatric/mediastinal mass characterization Off PTU or methimazole >3 d | 400 μCi I-123 sodium iodide via capsule or oral solution; pediatric 5.7 μC/kg; Uptake and scan: 4 h post images: anterior /anterior 10 cm/RAO, LAO, 5 min Uptake: 24 h Pinhole collimator, 159 keV 20% window Captus uptake probe |
| Thyroid uptake and scan – routine Off PTU or methimazole >3 d | 5–12 μCi sodium iodide I-131 capsule by mouth Adult dose: 6 mCi Tc-99m pertechnetate IV Returns 24 h after post I-131 for uptake measurement and then scan 20 min after injection Give crackers and water after injection Views: anterior, anterior −10 cm, RAO, LAO, 5 min/view Pinhole collimator, 140 keV, 20% window |
| Total body iodine scan No CT contrast within 4 wks Preparation options: 1) Discontinue levothyroxine for 4 wks 2) At 4 wks, switch from levothyroxine to liothyronine, then cease liothyronine at 2 wks prior to scan 3) Thyrogen™ | Adult dose: 2 mCi sodium I-123 oral solution Pediatric dose: 28.6 μCi/kg Patients on Thyrogen™: dosed at least 4 h after second Thyrogen™ shot Uptake w/Captus probe Images: whole body – anterior and post 8 cm/min; neck statics, neck statics w/sternal notch marker |
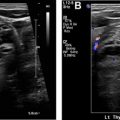
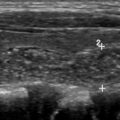
| Parathyroid scan | Adult dose: 25 mCi Tc99m-sestamibi Pediatric dose: 0.28 mCi/kg Images: immediate, anterior (10 min) Images: 2 h, anterior (10 min) |
| Parathyroid SPECT/CT |
|
Toxic multinodular goiter exhibits patchy uptake on scintigraphy, with a 24-hour radioiodine uptake of 30% to 60%. Although photopenic regions may represent degenerating adenomas or suppressed normal thyroid tissue, the “cold nodule” carries a 10% risk of malignancy and warrants ultrasound examination and potential biopsy. Thyroid malignancy is more common in patients with underlying thyroid disease and prior head and neck radiation. In cases of large nontoxic multinodular goiter when the patient is not a surgical candidate, recombinant human thyroid stimulating hormone (rhTSH: Thyrogen™, Genzyme Corporation, Cambridge, MA) can be used. In a hyperthyroid patient with a solitary tracer-avid focus and absent uptake elsewhere in the gland, this entity is likely a toxic adenoma. Toxic adenomas larger than 4 cm are usually surgically excised, but smaller nodules may be ablated successfully with 20 to 30 mCi I-131 sodium iodide (more activity is needed owing to suppressed gland and lower uptake). Toxic multinodular goiter is treated with a similar dose. After radioablative therapy, the failure rate is 10%, with underestimation of a large gland size representing the most common reason. A recent retrospective cohort study of 18,805 patients by Kitahara and colleagues showed a modest association between greater absorbed organ doses of radioactive iodine and increased risk of death from solid cancers, including breast cancer (5%–10% increase per 100-mGy dose).
Scintigraphic evaluation is occasionally performed for workup of neonatal hypothyroidism, which is usually secondary to thyroid dysgenesis or aplasia, or organification defect. For the latter, a perchlorate washout test may be performed using Tc99m pertechnetate and I-123; in this procedure, trapping of iodide is demonstrated on a Tc99m pertechnetate scan; however, a relative washout of more than 10% is observed on I-123 scans after administration of perchlorate, an inhibitor of iodide trapping (i.e., a sodium–iodine symporter), thereby confirming lack of organification. Additional use of I-123 sodium iodide scanning includes verification of the thyroid origin of indeterminate mediastinal masses.
Thyroid-malignant disease
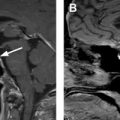
Thyroid cancer has increased in incidence, likely owing to increased detection, with more than 60,000 new cases in 2013, and is now the fifth most common cancer in women. Follicular epithelial-derived cancers (papillary, follicular, some poorly differentiated) concentrate iodine, enabling adjuvant treatment with I-131 sodium iodide, and express thyroglobulin, which may be used as a marker for tumor recurrence after ablation of remnant tissue after thyroidectomy. Follicular cancer is more aggressive and more likely to present with distant metastases than papillary histology ( Fig. 1 ). Hurthle cell carcinoma and anaplastic thyroid cancer are typically not iodine avid. Staging is either based on disease-specific mortality (American Joint Committee on Cancer) or the risk of recurrence (American Thyroid Association [ATA]). In the American Joint Committee on Cancer system, stages I to IV are based on T-, N-, and M parameters, with age less than 55 years being a positive factor and extrathyroidal extension, age greater than 55 years, and distant metastases being negative prognostic factors. In the 2009 ATA guidelines, high-risk patients may have distant metastases, gross extrathyroidal extension, or incomplete resection; intermediate-risk patients have nodal metastasis, advanced age, and adverse histology (e.g. tall cell); and low-risk patients have tumors confined to the thyroid, favorable histology, and no nodal or distant metastatic disease. After the issuance of the 2015 ATA guidelines, microscopic nodal metastases were placed into the low-risk category along with other changes. The 5-year survival of patients with localized tumor is 100%, nodal metastasis is 97%, and distant metastasis is 57%.

Factors associated with higher recurrence or mortality include advanced age, extrathyroidal extension, multiple and enlarged nodal metastases in the lateral neck, and distant metastatic disease (to the lungs or bones), and I-131 treatment is unquestionably the standard of practice for such patients. Its benefit in low-risk patients has not consistently been shown and radioablative therapy is now tending to be reserved for intermediate- or high-risk patients. Moreover, a recent large study demonstrated a slightly increased risk of salivary gland malignancies and leukemia with radioiodine therapy, which is dose dependent and inversely correlated with age.
Total body iodine scanning plays an integral role in the patient’s initial staging evaluation (see Table 1 ). After thyroidectomy, the patient is typically prepared by thyroid hormone withdrawal for 4 weeks, or Thyrogen™ 0.9 mg IM × 2 days (plus 1 week of a low-iodine diet) to achieve TSH stimulation, then administered 2 mCi I-123 sodium iodide, followed by a total body scan and uptake the following day.
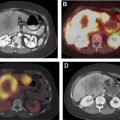
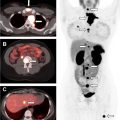
Diagnostic I-131 sodium iodide total body scan is less preferred, owing to concern of “stunning” the thyroid cancer cells and making them more resistant to subsequent radioiodine therapy. The total body scan may reveal unanticipated distant metastatic disease, prominent residual thyroid tissue that may warrant further surgery, or a burden of pulmonary metastases that may require dosimetry. A pretherapy scan is advocated by Van Nostrand and colleagues who, in a study of 355 scans, found that 53% of the patients had findings on preradioablation whole body scan that might have altered the management. Alternatively, the nuclear radiologist can treat empirically based on clinicopathologic data, and obtain a post-therapy scan 5 to 7 days later to complete the diagnostic evaluation; in the event of unanticipated metastatic disease, additional I-131 could still be given at a later time. Chen and colleagues also reported that I-123 preablation whole body scan was able to provide additional critical information in 25% of the patients, requiring significant changes in the I-131 therapy strategy. I-123 single photon emission computed tomography/computed tomography (SPECT/CT) scan can change management, most often on post-therapy scan, with a less clear benefit for the pretherapy scan. A SPECT scan is helpful for evaluating unexpected distant sites of iodine uptake ( Fig. 2 ).

Thyrogen™ is approved by the US Food and Drug Administration for remnant ablation, but is increasingly used off-label in patients with metastatic disease owing to increased convenience and patient comfort relative to thyroid hormone withdrawal. It has been demonstrated that renal clearance is lower with thyroid hormone withdrawal, suggesting that dose to tumor would be higher than with Thyrogen™. This finding is supported by studies of 124 I-PET/CT, which have showed a lower radiation dose to metastases with recombinant human TSH stimulation compared with thyroid hormone withdrawal and decreased detection of metastases compared with thyroid hormone withdrawal. ,
Other methods of treatment for thyroid cancer include thyroid hormone suppression, radiation, tyrosine kinase inhibition, and restoration of iodine avidity in iodine-negative cancers via the mitogen-activated protein kinase pathway (under investigation). I-124 PET/CT imaging has been used to quantitate difference in sodium-iodine symporter expression before and after such redifferentiation therapy in patients with radioiodine-refractory metastatic thyroid cancer.
State-of-the-art radioiodine therapy offers the opportunity to provide personalized dosimetry. In patients with renal impairment or diffuse pulmonary metastases ( Fig. 3 ), a pre-dosing and postdosing I-123 total body scan can be used to compute the effective renal clearance half-time, and using a modified Benua–Leeper approach, calculate maximum tolerated doses of I-131 sodium iodide to avoid bone marrow and lung toxicity, respectively. Sgouros and colleagues have suggested that the 80 mCi at 48-hour Benua threshold for lung toxicity may overestimate the risk of pulmonary complications. Lesion-specific dosimetry is possible using OLINDA software, but its use is not widespread.