Fig. 24.1
Different types of vulnerable plaque as underlying cause of acute coronary events and sudden cardiac death. (a) Rupture-prone plaque with large lipid core and thin fibrous cap infiltrated by macrophages. (b) Ruptured plaque with subocclusive thrombus and early organization. (c) Erosion-prone plaque with proteoglycan matrix in a smooth muscle cell-rich plaque. (d) Eroded plaque with subocclusive thrombus. (e) Intraplaque hemorrhage secondary to leaking vasa vasorum. (f) Calcific nodule protruding into the vessel lumen. (g) Chronically stenotic plaque with severe calcification, old thrombus, and eccentric lumen. Reproduced with permission from Naghavi et al. [5]
The identification of these vulnerable plaques is expected to improve the prediction of future cardiovascular events. The noninvasive imaging techniques currently available in clinical practice are, besides identifying the degree of lumen stenosis, capable of evaluating plaque size, plaque morphology, and a number of plaque components. However, especially in the early stages of development the size of most plaque components is too small to accurately image with the current spatial resolution [8, 9]. To date the identification of individual plaque components has not yet resulted in a clinically relevant improvement in prediction [10].
The use of molecular imaging to identify biological processes associated with plaque development and vulnerability could further improve prediction of cardiovascular events. Additionally molecular imaging could detect plaque in an earlier phase of development and provide new insights into the natural history of atherosclerotic disease, and aid the development of new therapies by target selection and validation in vivo, and monitoring of treatment effects [11–13].
The members of the Molecular Imaging Center of Excellence Standard Definitions Task Force have defined molecular imaging as the visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans or other living systems [14]. Jaffer and Weisleder have formulated four key questions that need to be addressed before molecular imaging can be applied [15].
1.
Is there a molecular target relevant to the disease of interest?
2.
Once the target is selected, is there a high-affinity ligand that will bind to the target?
3.
What is the appropriate molecular imaging modality to provide the required spatial resolution, sensitivity, and depth penetration for the disease?
4.
For a given imaging modality, can a molecular imaging agent be synthesized to detect the desired molecular target?
This chapter will review the use of noninvasive molecular imaging for the detection of inflammation and intraplaque neovascularization, two closely related and essential factors in plaque vulnerability. We will focus on the challenges in molecular imaging using nuclear imaging, magnetic resonance imaging (MRI), ultrasound, computed tomography (CT), and multimodality imaging for the evaluation of vulnerable plaques. Implementation of these modalities, for both molecular imaging and molecular guided therapy, will be addressed.
2 Inflammation and Intraplaque Neovascularization
Several molecular targets involved in atherosclerosis have been identified in basic research [16]. Inflammation and neovascularization are of special interest for molecular imaging due to their expression of endothelial markers in the lumen. Many of the molecular contrast agents are relatively large and are therefore restricted to intraluminal targets. The intraluminal expression of molecules associated with neovascularization and inflammation allows for easy targeting. Furthermore, their involvement in the earliest stages of plaque development makes them interesting targets for early detection of plaque development, even before intimal thickening occurs.
The development of an atherosclerotic plaque has long been thought of as a disease originating from the lumen–intima border, initiated by the diffusion of lipids and transportation of monocytes over the luminal endothelium. However, in recent years evidence has been gathered to support the role of the adventitial side of the vessel wall, especially the vasa vasorum, in plaque development [17]. The vasa vasorum form a microvascular network in the vessel wall of large arteries that provides the vessel wall with oxygen and nutrients. The vasa vasorum have been reported to provide an additional endothelial surface area of 17% of the main lumen endothelial surface in normal coronary arteries [18]. This number can rise to about almost 70% in regions with non-calcified plaques [18, 19].
All cells in the human body are dependent on the vascular system for delivery of oxygen and nutrients. The majority of cells of the arterial vessel wall obtain their oxygen and nutrients by diffusion from the main lumen. However, in vessel walls more than 29 lamellar units thick, diffusion from the lumen is no longer sufficient. In these arteries the delivery to the adventitia and outer media is supplemented by vasa vasorum [20–24].
In humans vasa vasorum are present in all arteries with a vessel wall thickness >0.5 mm [24]. The majority of vasa vasorum originate from side branches of the main artery and enter the arterial wall from the abluminal side (vasa vasorum externa; Fig. 24.2), though vasa vasorum originating directly from the main lumen (vasa vasorum interna) are present as well. Additionally a network of venous vasa vasorum has been identified that drains to veins in proximity to the artery [25]. In a normal vessel the vasa vasorum are restricted to the adventitia and outer parts of the media. However, in atherosclerotic vessels neovascularization sprouting from the vasa vasorum into the intimal parts of the plaque has been found [26, 27]


Fig. 24.2
Sketches of the three types of vasa vasorum found in the wall of cow aortae [inspired by Schoenenberger and Mueller 157]. In the Schoenenberger and Mueller study, vasa vasorum interna (left panel) originated directly from the aorta’s main lumen, and vasa vasorum externa (mid panel) originated from intercostal branches deriving from the main lumen and dived back into the aortic wall. Venous vasa vasorum (right panel) developed in the aortic wall and finally drained into branches of concomitant veins. Reproduced with permission from Gössl et al. [25]
Intraplaque neovascularization has gained interest as a factor in the development, progression, and vulnerability of atherosclerotic plaques. Pathologic studies have shown that the presence of intraplaque neovascularization, especially in the plaque shoulder where the plaque is most vulnerable to rupture, is associated with vulnerable and symptomatic plaques [18, 26–28]. Additionally, the presence of intraplaque neovascularization has been found to be an independent predictor of intraplaque hemorrhage and plaque rupture [27, 29]. Hellings et al. [30] investigated whether plaque composition is associated with the occurrence of future cardiovascular events. Endarterectomy specimens were collected for histology and patients were followed for 3 years after endarterectomy. Patients with intraplaque neovascularization or intraplaque hemorrhage were found to be at increased risk for a combined endpoint of cardiovascular events (fatal or nonfatal stroke, fatal or nonfatal myocardial infarction, sudden death or other vascular death) and any arterial vascular intervention not planned at time of inclusion (Fig. 24.3). The presence of macrophages, a large lipid core, calcifications, collagen, or smooth muscle cells was not associated with clinical outcome [30].
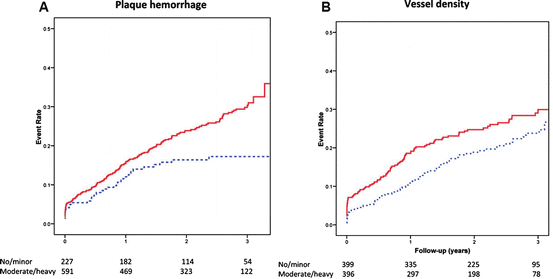
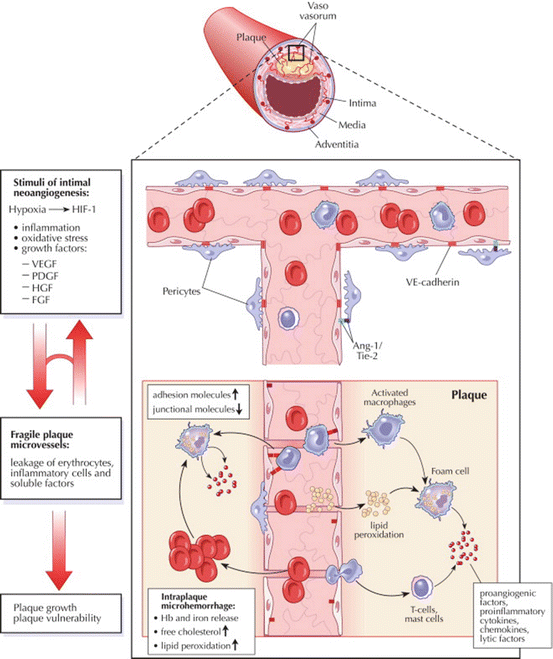

Fig. 24.3
Kaplan–Meier survival curves of plaque histology vs. primary outcome (vascular event and vascular intervention) for plaque hemorrhage (a): absent (dashed line) vs. present (continuous line) and intraplaque vessel density (b): <8 (average number of vessels per hot spot, dashed line) vs. ≥8 vessels (continuous line). The number of patients at risk for systemic cardiovascular events at 0, 1, 2, and 3 years is provided. Reproduced with permission from Hellings et al. [30]

Fig. 24.4
Plaque microvessels show a compromised structural integrity and a modified expression profile of adhesion and junctional molecules, thus permitting extravasation of inflammatory cells and soluble factors as well as the occurrence of microhemorrhages. The resulting reactive microenvironment may support further plaque growth and plaque vulnerability. Ang angiopoietin, FGF fibroblast growth factor, Hb hemoglobin, HGF hepatocyte growth factor, HIF hypoxia-inducible factor, PDGF platelet-derived growth factor, VE vascular endothelial, VEGF vascular endothelial growth factor. Figure illustration by Rob Flewell. Reproduced with permission from Mause and Weber [31]
The association between intraplaque neovascularization, intraplaque hemorrhage, and cardiovascular events is thought to be due to the poor structural integrity of the newly formed vasa vasorum [31]. The majority of neovessels in symptomatic plaques are highly irregular in shape, while very few of these irregular vessels are present in asymptomatic plaques [32]. The newly formed microvessels are thin-walled, with incomplete or absent endothelial gap junctions. This may result in the~extravasation of lipids, inflammatory cells, and red blood cells, and risks the collapse of microvessels, causing intraplaque hemorrhage (Fig. 24.4). This will contribute to lipid core growth and sustained plaque inflammation, thus the progression into more advanced plaques [33, 34]. Consequently plaques with a high density of neovascularization are at an increased risk of plaque rupture [35, 36].
Angiogenesis, the sprouting of new microvessels from an existing microvascular network, is the main process resulting in neovascularization of atherosclerotic plaques [35, 37]. Key factors initiating angiogenesis in atherosclerosis are still largely unknown. Developments in tumor research have greatly increased our knowledge about the processes that take place during neovascularization and identified several factors that can initiate neovascularization such as metabolic stress (hypoxia, acidosis, or hypoglycaemia), mechanical stress (pressure generated by proliferating cells), immune/inflammatory response (immune/inflammatory cells that have infiltrated the tissue), and genetic mutations [38].
Hypoxia has been found to be one of the most important stimuli for angiogenesis in several diseases [39, 40]. Upregulation of hypoxia-inducible factors has been found in atherosclerotic plaques [41, 42]. Though hypoxia may arise due to the increasing distance between the vessel lumen and plaque core, this is unlikely to be the main culprit, since angiogenesis is already present in early stages of plaque development when intimal thickening is negligible [43–46]. Hypoxia is thought to be primarily determined by the increased oxygen demand caused by plaque inflammation [35].
Inflammation is one of the hallmarks of plaque development and vulnerability and inflammatory cells have generally been accepted to play a key role in the early stages of plaque formation. In the early stages the vessel wall is infiltrated by lipoproteins and as a response the endothelium expresses leukocyte adhesion molecules. Monocytes in the lumen adhere to the adhesion molecules and extravasate into the subintimal space [47]. The influx of monocytes and their subsequent differentiation into mature and activated inflammatory cells increases the metabolic demand in the vessel wall. If the supporting vasculature is insufficient this will result in a hypoxic state and increased oxidative stress. This hypothesis has been supported by the presence of both hypoxic and inflammatory factors, such as hypoxia-inducible factor-1α (HIF-1α), nuclear transcription factor-kappa B, tumor necrosis factor-α, and interleukin-6 and an increased number of superoxide anions in atherosclerotic plaques. Furthermore, these factors have been found to be co-localized with intraplaque neovascularization [41, 48, 49].
3 Imaging Modalities
The noninvasive imaging modalities currently utilized in clinical practice are after the administration of a specialized contrast agent (e.g., iron oxide, gadolinium, microbubbles, radionuclides, iodine, gold, bismuth) capable of molecular imaging (Table 24.1).
Table 24.1
Brief comparison of technological features of noninvasive imaging modalities used in clinical practice
Imaging modality | Spatial resolution (mm) | Acquisition time/frame (s) | Sensitivity of detection (g/ml) | Anatomical detail | Plaque composition | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
PET | 3–8 | 1–300 | <10−12 | − | − | Quantitative measurements | Ionizing radiation |
Short half-life tracers | |||||||
Expensive equipment | |||||||
Requires on-site cyclotron | |||||||
SPECT | 5–12 | 60–2,000 | >10−9 | − | − | Multiple isotope imaging | Ionizing radiation |
MRI | 0.1–0.2 | 50–3,000 | >10−6 | ++ | +++ | No ionizing radiation | Contrast-induced systemic fibrosis |
Widely available | |||||||
Ultrasound | 0.1–1.0 | 0.05–1 | NA | + | + | No ionizing radiation | Limited penetration depth |
Widely available | Obligatory intravascular contrast agent | ||||||
Inexpensive | |||||||
Real-time imaging | |||||||
CT | 0.5–1 | 1–300 | >10−3 | +++ | + | Coronary imaging | Ionizing radiation |
Widely available | Contrast nephrotoxicity |
Nuclear Imaging
Nuclear imaging includes a number of techniques that are all dependent on a physical signal emitted by a radionuclide tracer. The two predominant techniques currently in use are positron emission tomography (PET) and single photon emission computed tomography (SPECT). Both techniques have a high penetration depth and are capable of 3D whole body scanning.
Inherent to the dependency on a physical signal is the very high sensitivity with which nuclear imaging can detect the molecular tracer [50]. The main drawbacks of nuclear imaging are the limited spatial resolution, and the lack of anatomical information. This is partially overcome by co-registration with either CT or MRI to provide high-resolution anatomical information. The use of radionuclide tracers has the inherent problem of radiation exposure of both personnel and patient. Though the radiation doses currently used in clinical practice are safe, the effect of cumulative radiation exposure should be taken into account when performing repeated measurements.
Magnetic Resonance Imaging
MRI molecular imaging is performed using either gadolinium or superparamagnetic iron oxide compounds (SPIOs) that influence the magnetic resonance signal. MRI provides a sub-millimeter spatial resolution and good soft tissue contrast, thus providing an excellent evaluation of anatomical structures. In recent years increasing field strengths and improved coil designs have resulted in higher signal-to-noise ratios, contrast-to-noise ratios, and resolutions, while reducing scanning times. Additionally MRI can obtain anatomic, physiologic, and metabolic information in one session.
Ultrasound
B-mode and Doppler ultrasound are the most frequently utilized imaging modalities in clinical practice. Ultrasound has a high spatial resolution, which provided excellent anatomical information. Ultrasound contrast agents consist of gas-filled microbubbles stabilized by a lipid or protein shell. For molecular imaging ligands can be bound to these microbubbles. When using specific pulse sequences ultrasound is capable of specific contrast imaging, which eliminates the signal of the surrounding tissue. This makes ultrasound very sensitive for the detection of contrast agent [53]. Untargeted ultrasound contrast agents have been used in echocardiography for decades and have been proven safe in large multicenter studies [54, 55].
Ultrasound has a superior temporal resolution, which makes it the only technique currently available for real-time imaging. However, the penetration depth and 3D scanning capabilities are limited. Another important limitation is the relatively large size (1–5 μm) of the microbubbles, making them obligatory intravascular tracers. However, this makes them well suited for identifying the presence of vasculature, since the presence of contrast agent indicates the presence of a vessel.
Computed Tomography
CT (especially the latest generations of multislice and dual source CT) provides a high-resolution image with very short acquisition times, good penetration depth, and full body scanning capabilities. CT is currently the only noninvasive technique in clinical practice with sufficient resolution, penetration, and speed to accurately image the coronary arteries in a beating heart. CT provides good anatomical information; however, it is poor in soft tissue contrast. However, the use of CT for molecular imaging of the coronary arteries has not yet been investigated.
Molecular CT contrast agents are in early stage of development. Molecular contrast agents based on iodine, gold, and bismuth have been developed; however, they have not yet been investigated in humans [56–59]. The use of ionizing radiation potentially limits the use of CT, though the radiation dose in cardiovascular imaging has substantially been reduced in recent years [60]. From clinical practice it is known that iodine contrast agents can induce a contrast nephropathy, limiting its use in patients with decreased renal function [61].
Multimodality Imaging
is being explored to improve the accuracy of examinations. The currently available modalities each have their limitations as previously described. When using multiple imaging modalities the advantages of each individual modality can be used to the other modalities’ limitations, in order to improve the overall accuracy. In clinical practice multimodality imaging is already used by combining nuclear imaging with co-registration of either CT or MRI. This allows for the combination of the highly accurate and quantifiable assessment of biological activity by nuclear imaging with the high-resolution anatomical information provided by CT or MRI (Fig. 24.5) [62, 63].
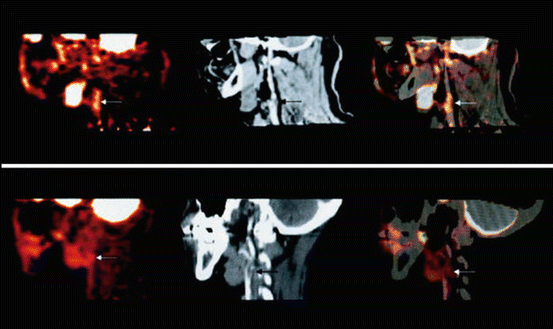
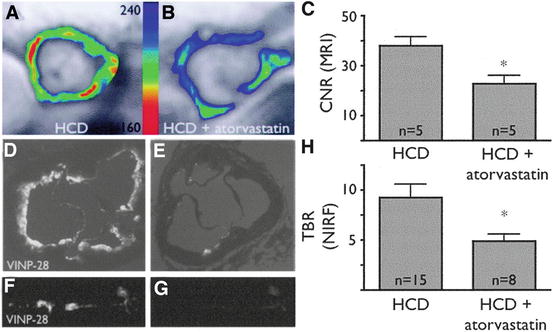

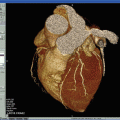
Fig. 24.5
The upper row (from left to right) shows PET, contrast CT, and co-registered PET/CT images in the sagittal plane, from a 63-year-old man who had experienced two episodes of left-sided hemiparesis. Angiography demonstrated stenosis of the proximal right internal carotid artery; this was confirmed on the CT image (black arrow). The white arrows show FDG uptake at the level of the plaque in the carotid artery. As expected, there was high FDG uptake in the brain, jaw muscles, and facial soft tissues. The lower row (from left to right) demonstrates a low level of FDG uptake in an asymptomatic carotid stenosis. The black arrow highlights the stenosis on the CT angiogram, and the white arrows demonstrate minimal FDG accumulation at this site on the FDG-PET and co-registered PET/CT images. Reproduced with permission from Ross et al. [93]

Fig. 24.6
Noninvasive MRI assessment of VCAM-1 expression after atorvastatin administration. (a) Short-axis MRI of the aortic root of an untreated apoE−/− mouse on a high cholesterol diet (HCD) after injection of a VCAM-1-targeted nanoparticle (VINP-28) with color-coded signal intensity. The red color encodes high VCAM-1 expression. (b) MRI of HCD + atorvastatin-treated apoE−/− mouse after VINP-28 injection. An attenuated signal drop in the aortic root wall compared with untreated mouse was noted. (c) After injection of VINP-28, the MRI contrast-to-noise ratio diminished in atorvastatin-treated mice (mean ± SD; *P < 0.05 versus HCD). (d, e) Near-infrared (NIR) microscopy of the aortic roots depicted in a and b. Fluorescent signal originating from VINP-28 comprised the whole-root circumference (d), but less so in atorvastatin-treated mice (e). (f, g) Fluorescent reflectance imaging of the excised aorta of an untreated (f) and atorvastatin-treated (g) mouse showed reduced NIR signal in statin-treated mice. (h) The target-to-background ratio was reduced in aortas of treated mice (mean ± SD;*P < 0.05 treated vs. untreated mice). Reproduced with permission from Nahrendorf et al. [69]
The use of multimodal contrast agents is currently limited to preclinical research. A number of probes have been developed that enable both in vivo and ex vivo molecular imaging (e.g., MRI and fluorescence [64]). However, probes that are detectable with multiple in vivo imaging modalities (e.g., MRI and PET [65]) are sparse. The development of multimodal probes could help achieve high sensitivity and high spatial resolution.
4 Molecular Targets
Leukocyte Adhesion Molecules
The expression of leukocyte adhesion molecules is a marker of endothelial dysfunction and is pivotal in the initiation of vessel wall inflammation [47]. Since adhesion molecules are expressed in the vascular lumen they are easily accessible to molecular contrast agents. Frequently investigated adhesion molecules include vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and selectins. VCAM-1 and ICAM-1 and selectins are expressed by the luminal endothelium overlying the atherosclerotic plaque. However, the most dominant expression has been found at the adventitial site of the plaque and correlated with sites of vasa vasorum neovascularization, vascular permeability, and a high number of inflammatory cells [66, 67].
VCAM-1-targeted MRI contrast agents have utilized VCAM-1’s ability to internalize ligands, resulting in accumulation of the contrast agent. In vivo VCAM-1-targeted magnetofluorescent nanoparticles were shown to be able to identify VCAM-1 expression in atherosclerotic lesions by MRI, which was confirmed by fluorescence imaging and histology [68, 69]. In ex vivo endarterectomy specimens it was confirmed that the VCAM-1-targeted contrast agents co-localized with VCAM-1 expressing endothelial cells in human plaques as well [69]. Additionally it was shown that statin therapy reduced the contrast enhancement caused by the VCAM-1-targeted contrast on MRI and fluorescence imaging (Fig. 24.6), which correlated with reduced expression of VCAM-1 on histology [69]. (Table 24.2).
A VCAM-1-targeted contrast agent has also been developed for ultrasound [70]. In vitro experiments showed that VCAM-1-targeted microbubbles were able to attach to activated murine endothelial cells, even under a pulsatile high shear stress condition. Subsequent in vivo experiments showed ultrasound signal enhancement in the aortic plaques of apoE-deficient mice within 10 min after contrast injection [70]. These results suggest that VCAM-1-targeted microbubbles could provide a fast molecular imaging technique. The availability of a fast molecular imaging technique could be advantageous for clinical practice.
Similarly, ICAM-1-targeted microbubbles were shown to specifically bind to endothelial cells expressing ICAM-1 in vitro [71]. In vivo ICAM-1-targeted microbubbles were found to attach to what are described as early stages of the atherosclerotic plaque (Fig. 24.7) [72]. Though this study showed that ultrasound might be capable of identifying the early stages of atherosclerotic vascular disease, no histology was performed to confirm this finding.
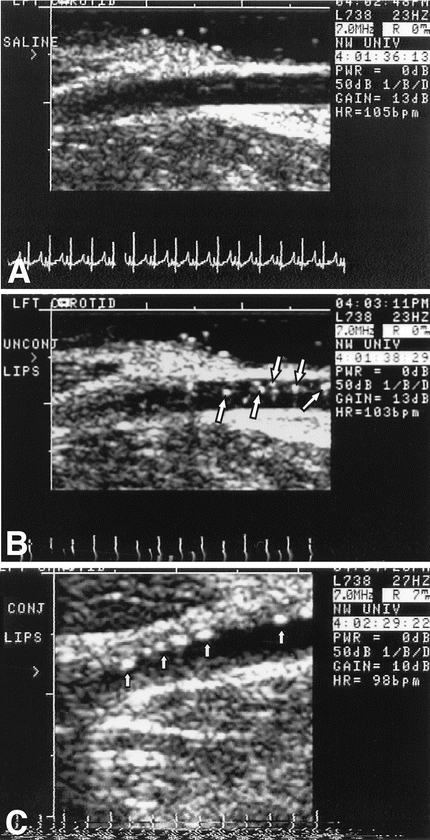
Table 24.2
Available studies on potential molecular targets involved in neovascularization and inflammation of the atherosclerotic plaque
Target | Nuclear imaging | MRI | Ultrasound | CT | In vivo imaging | Atherosclerosis model |
|---|---|---|---|---|---|---|
Leukocyte adhesion molecules | ||||||
VCAM-1 | Animal | Yes | ||||
ICAM-1 | [155] | Animal | Yes | |||
e-Selectin | [133] | Animal | No | |||
p-Selectin | [75] | No | Yes | |||
Inflammatory cells | ||||||
Phagocytosis | [56] | Animal | Yes | |||
Macrophage metabolism | Animal | Yes | ||||
Human | Yes | |||||
MMP | [103] | [102] | Animal | Yes | ||
Myeloperoxidase | [104] | Animal | Yes | |||
Angiogenesis | ||||||
VEGFR2 | Animal | No | ||||
Endoglin | [112] | [113] | Animal | No | ||
Integrin αvβ3 | Animal | Yes | ||||
Human | No | |||||
Fibronectin ED-B | Animal | No | ||||
Human | No | |||||

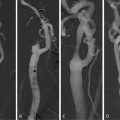
Fig. 24.7
Transvascular ultrasound images of an atherosclerotic left carotid artery of a Yucatan miniswine. (a) After injection of saline. (b) After injection of unconjugated liposomes (arrows point to liposomes within the lumen). (c) After injection of anti-ICAM-1-labeled liposomes (arrows point to liposomes attached to the atherosclerotic plaque). Reproduced with permission from Demos et al. [72]
Selectin-targeted contrast agents have been developed but not yet investigated for the imaging of atherosclerotic plaques. Both e-selectin- and p-selectin-targeted SPIO compounds have shown binding to human endothelial cells in vitro and a concomitant significant T2 signal decrease on MRI [73–75]. e-Selectin-targeted MRI contrast agents have been used in vivo to identify inflammation in hepatic and muscle tissue of mice [76, 77].
Inflammatory Cells
The activated endothelium releases chemoattractants to recruit monocytes. These subsequently enter into the sub-endothelial space where they differentiate in mature inflammatory cells. Macrophages are the most prevalent inflammatory cell in the atherosclerotic plaque and play a major role in the pathophysiology of plaque vulnerability [78]. The macrophages can cause a maladapted chronic inflammatory response that will aid the expansion of the sub-endothelial layer due to the accumulation of cells, lipids, and matrixmolecules [78]. The presence of macrophages in the plaque has been found to be closely associated with intraplaque neovascularization [79]. Macrophages both use the vasa vasorum neovascularization as a port of entry into the plaque and excrete a myriad of proangiogenic molecules aiding the expansion of the microvascular network.
Macrophages provide a number of attractive targets for molecular imaging. Their ability to phagocytose molecules can be utilized to accumulated contrast agent. This should improve the target-to-background ratio and thus the accuracy with which contrast agent can be detected. Activated macrophages have been shown to internalize and concentrate a number of nanoparticles. For example, in vitro macrophages have been shown to actively phagocytose dextran-coated SPIO nanoparticles under the influence of cytokines, serum components, and statin treatment [80]. Similarly, both gadolinium micelles and iodinated nanoparticles have been shown to be internalized by macrophages [56, 81].
Dextran-coated SPIO nanoparticles have been investigated in human endarterectomy patients. The SPIO nanoparticles were shown to be to be able to identify macrophages in vivo [82–86]. However, due to the long interval between pre- and post-contrast scans (≥24 h) the clinical application of SPIO nanoparticles seems limited.
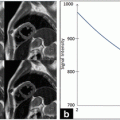
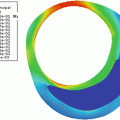
The Atorvastatin Therapy: Effects on Reduction of Macrophage Activity (ATHEROMA) Study investigated the effects of low-dose (10 mg) and high-dose (80 mg) atorvastatin on carotid plaque inflammation as determined by ultrasmall SPIO compound. The primary endpoint was the change in signal intensity from baseline after 6 and 12 weeks of either low-dose or high-dose atorvastatin. Both at 6 and 12 weeks there was a significant reduction in signal intensity from baseline in the high-dose group (Fig. 24.8). There was no difference observed in the low-dose group [87].
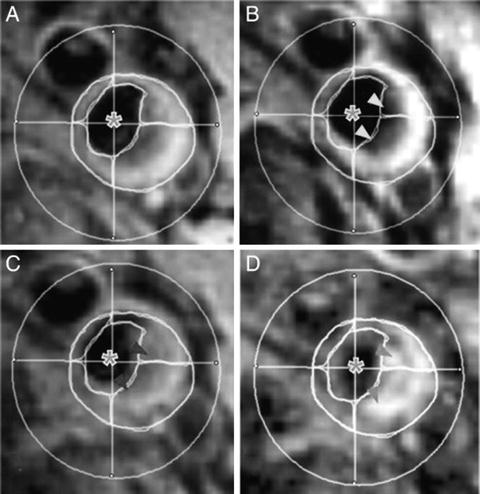

Fig. 24.8




T2*-weighted MRI images of the right common carotid artery of a patient receiving 12 weeks of high-dose atorvastatin (80 mg) treatment. Imager were taken before (left) and after (right) ultrasmall superparamagnetic iron oxide (USPIO) infusion at time points 0 (a and b) and 12 weeks (c and d). (b) USPIO uptake can clearly be seen in the plaque at baseline (yellow arrowheads). (c) USPIO has been cycled out of the plaque before reinfusion at 12 weeks (red arrowheads). (d) The plaque enhances at 12 weeks (blue arrowheads), indicating that the high-dose statin treatment has damped the USPIO-defined inflammation. Plaque segmentation was performed using a combination of multicontrast sequences. The cross hairs centered through the middle of the lumen divide the vessel into quadrants. Signal from artifact, extravascular structures, and the luminal blood pool (green asterisks) are excluded from the analysis. Reproduced with permission from Tang et al. [87]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree