, Yves Etienne2 and Maurice Niddam3
(1)
Faculté de Médecine, Marseille, France
(2)
Unité de Médecine Légale, Hôpital de la Timone, Marseille, France
(3)
Unité SAMU 13, Centre 15, Hôpital de la Timone, Marseille, France
4.1 Macroscopic Aspect
The amygdaloid body or cerebral amygdala appears as a small disc-shaped formation, more or less convex, which caps the top surface of the head of the hippocampus and thus forms thereon a discrete cup-shaped cavity (Fig. 4.1).
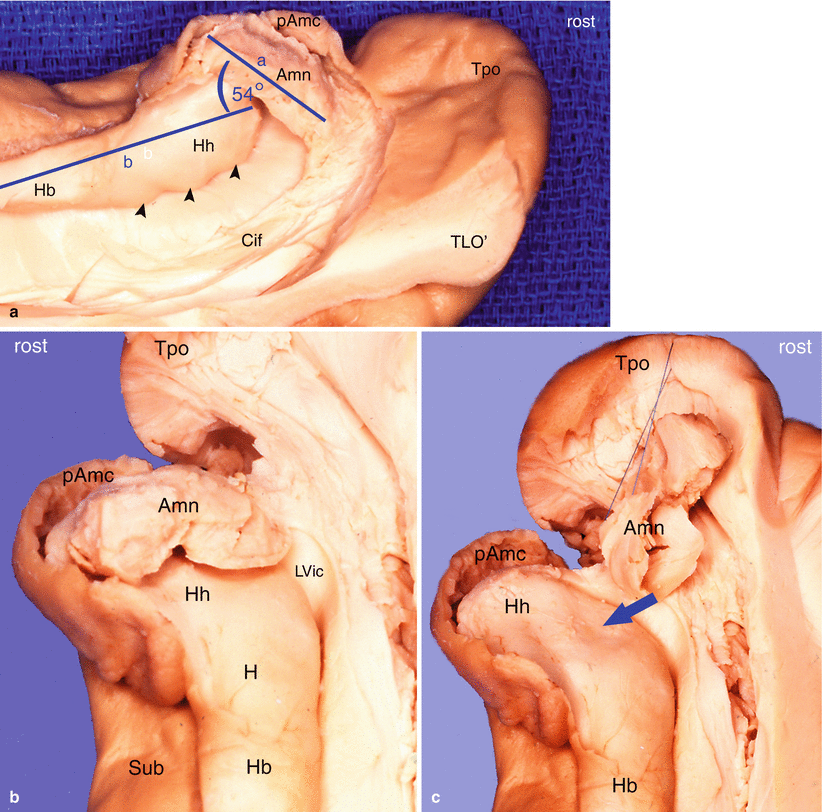

Fig. 4.1
Relations of the amygdaloid nuclear complex with the hippocampal head. rost: rostral. (a) supero-medial view of the temporal lobe’s anterior part. (b) superior view of the temporal lobe’s anterior part. (c) The same view as b, with separation of the Amn (lifted). Amn amygdala, Cif cingulate fasciculus, H hippocampus, Hb hippocampus body, Hh hippocampus head, pAmc periamygdalar cortex, LVic lateral ventricle, inferior cornu, sub subiculum, TLO’ sagittal section of the temporal lobe, blue arrow showing the cupuliform print made by the amygdala’s convexity, black arrowheads digitationes hippocampi (internal digitations). Observe on (a): The great axis of the amygdala and the hippocampal bodies’axis (blue coloured) form an angle of 54°
The morphology of the amygdala is most variable, its surface being dented in a variable manner by the surfacing of its constitutive nuclei. However, we can retain an overall disc-shaped appearance with two slightly convex surfaces. The almond shape described by the ancient authors provides an approach of the morphological reality. However, many amygdalae are hardly dished and have the aspect of a flattened disc, which resembles a flat dragee or a little plate haricot bean. Conversely, some amydalas do not match the above-mentioned comparisons (Fig. 4.2): they are the globular and polylobed amygdalae, clusters of nuclei rather than a unitary organ.
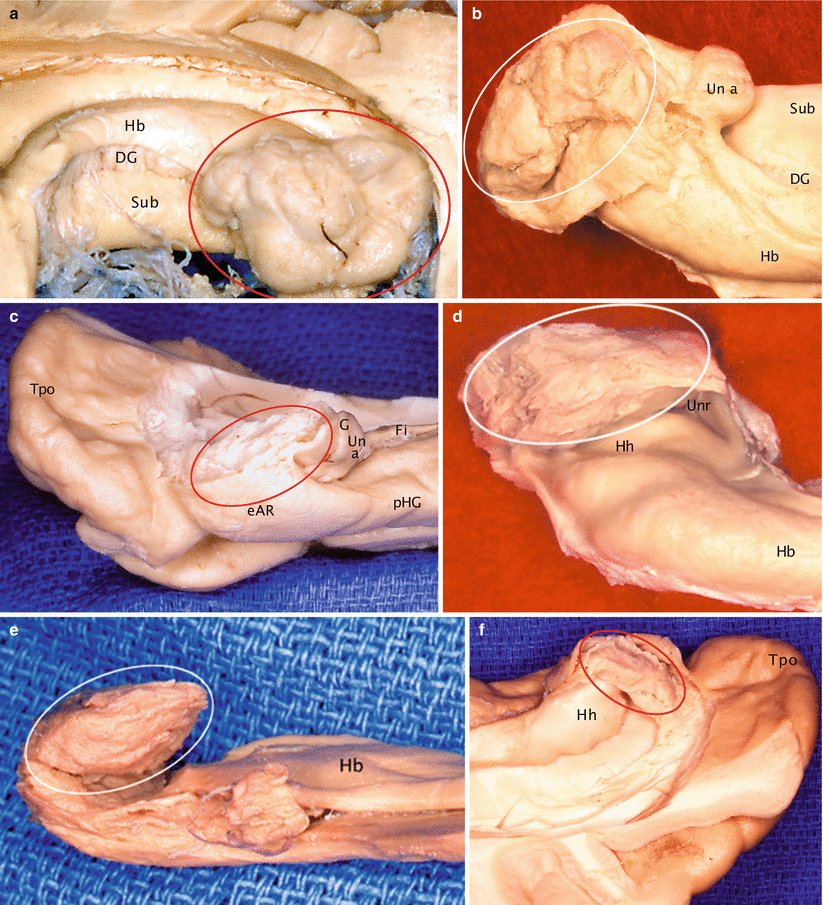

Fig. 4.2
Morphological aspects of the amygdala, in situ, above the hippocampal head. (a) Globular with bulging nuclei; (b) reniform; (c) flattened; (d) massive; (e) classical, almond shaped; (f) in shape of a butter’s package. DG dentate gyrus, eAr entorhinal area, Fi fimbria, G band of Giacomini, Hb hippocampal body, Hh hippocampal head, pHG parahippocampal gyrus, Sub subiculum, Tpo temporal pole, Un a uncal apex, Unr uncal recess. On every picture of the artwork, the amygdala is surrounded by a circle so as to be displayed well
The colour of the amygdala is that of the grey substance. When a brain section passing through the amygdala is stained by using our ferric chloride staining process, it is observed that the amygdala is coloured with the central grey nuclei but with different shades: the amygdala stains less intensely than the caudate nucleus or the putamen but more intensively than the pallidum and thus evokes a specific neurochemistry. Some amygdalae are very difficult to colour, and we cannot see why!
The inclination of the amygdala is characteristic: it more or less lies on the head of the hippocampus. If we look at the two formations on the medial dissections of hemispheres, it is observed that the major axis of the amygdala forms, with the long axis of the body of the hippocampus, an average angle ranging from 30 (for the most horizontal) to 45° for the most vertical (Fig. 4.1).
The amygdala, in such a position, especially if it is disc shaped, has a dorsal, antero-superior surface directed outwards and a postero-inferior surface facing the head of the neighbouring hippocampus directed slightly inwards (Figs. 4.3, 4.4 and 4.5).
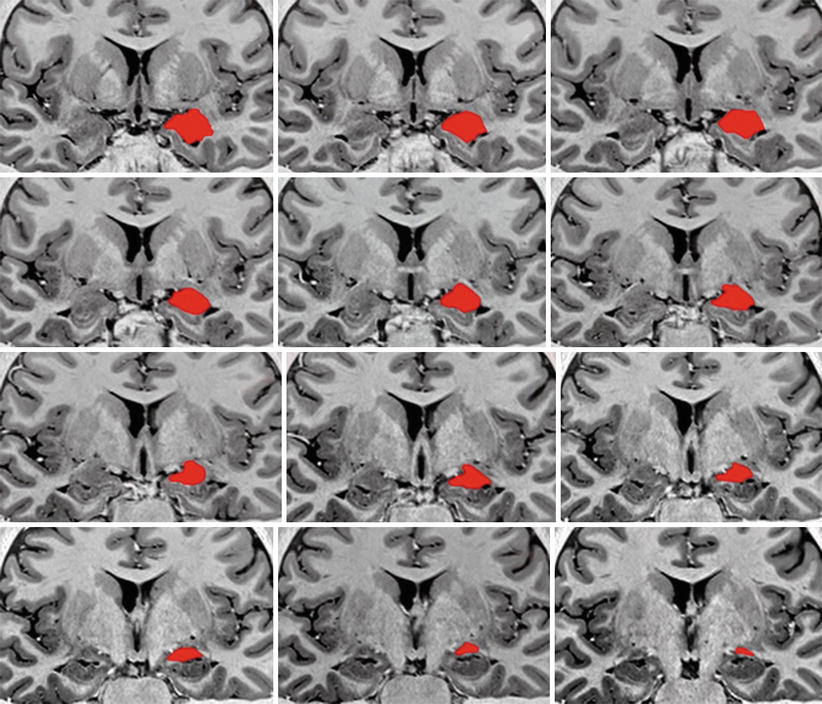
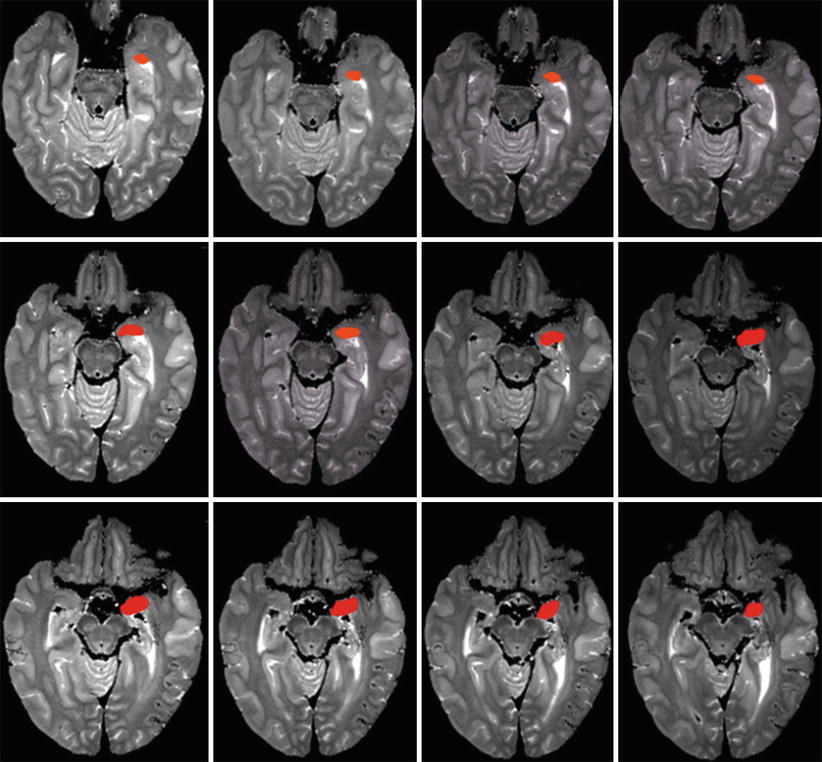
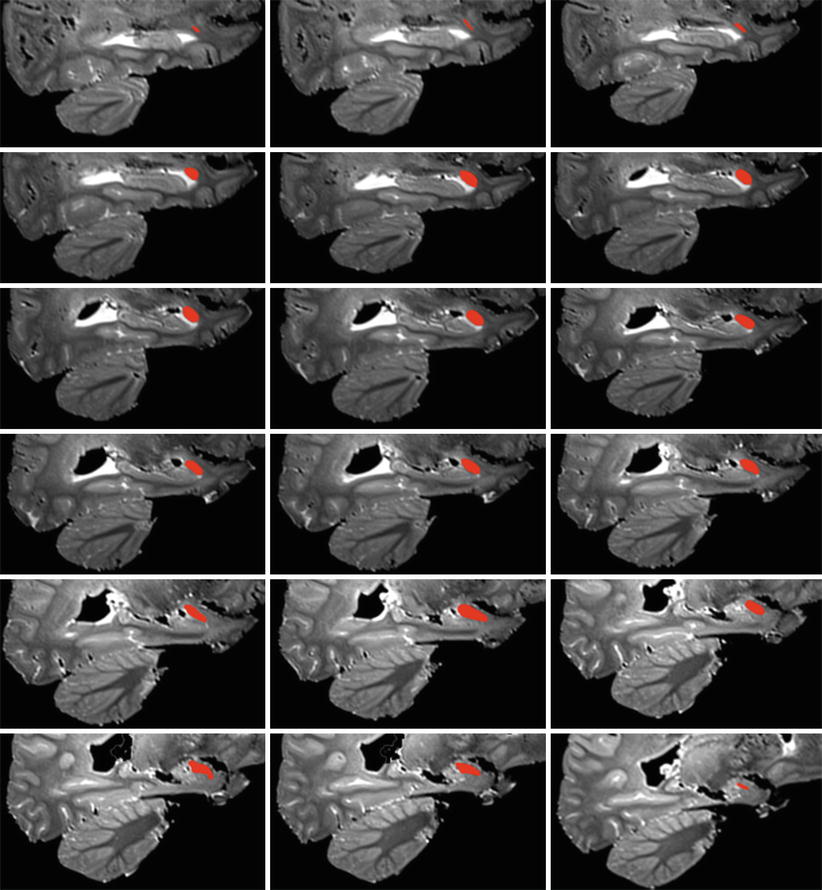

Fig. 4.3
A series of successive MRI coronal sections of the brain with red coloured left amygdalae in order to show their coronal main lines. The slightly oblique main lines at the top of the picture (the more anterior sections) become gradually more or less horizontal on the posterior sections

Fig. 4.4
A series of successive MRI axial sections of the brain with red coloured left amygdalae in order to show their axial main lines. The more or less horizontal main lines at the lower level of the sections (top of the picture) become gradually oblique behind and inside at the higher sections

Fig. 4.5
A series of successive MRI sagittal sections of the brain with red coloured left amygdalae in order to show their sagittal main lines. The main line remains oblique below and forwards at all the sagittal sections
The average measurements that we have observed during our dissections clearly show that there are differences between individuals but also slight right–left differences, on the same individual (measurement differences resulting from different morphologies). The length of the major axis varies between 12 and 19 mm; the greatest width varies between 6.5 and 9 mm; the average thickness varies between 1 and 4 mm.1 The weight is usually less than 1 g, with average values between 0.56 and 0.69 g.
Appreciating the volume of the amygdala raises many problems.
It can be easy if it concerns amygdalae obtained by anatomical sampling, and therefore cadaveric amygdalae, keeping in mind that this type of sampling campaign is only conducted on the nuclear complex and therefore eliminates the periamygdaloid cortex.
In our experience, we found with this method an average figure of 1100 mm3. We observed no significant difference between the volumes of the two amygdalae from the same subject. However, we did observe that male amygdaloid volumes are slightly greater than female amygdaloid volumes, but there again without any major significance due to the fact that men generally have larger brains. J Brabec et al 2010 also carried out anatomical volume measurements based on the flatness of serial sections stained by using the Nissl method. They obtained an average volume of 1.24 cm3 when not taking into account the circumference of the amygdala and 1.63 cm3 if this circumference is included. These authors have not observed any intersexual or interhemispheric difference.
The observation of the amygdaloid volume in living subjects is even more interesting. It was made possible by the three-dimensional reconstruction processes using MRI (magnetic resonance imaging). However, once again, difficulties arose due to discrepancies in the landmarks that the writers adopt as contours of the amygdala. This explains that the results vary according to the authors: 1154 mm3 (right side) to 1160 mm3 (left side), for JC Pruessner et al. 2000, but 1691.7 mm3 (right side) to 1726.7 mm3 (left side), for B Brierley et al. 2002. The divergence in the results is regrettable. That is why, in 1992, C Watson et al. 1992 attempted to standardise the measurements based on anatomical criteria, which are referred to since then as “Watson’s criteria”. But besides the fact that these criteria were complex to handle and therefore difficult to implement and that they generated numerical results exceeding standard averages, it is the development of new segmentation protocols (JL Hanson et al. 2012; JJ Entis et al. 2012) which should now ensure a more reliable numerical analysis of MRI images and provide comparable volumetric results whatever teams are conducting these measurements. Being able to accurately measure, as a routine process, the volume of the amygdala would be very interesting since some volume variations are due to dysfunctions of the nervous system.
Thus, C Lange and E Irle 2004 observed amygdalae with an increased volume in young women who had recently suffered from a major depression. JP Hamilton et al. 2008 only noted this increase in treated patients (antidepressants seem to secrete neurotrophic factors favouring neurogenesis!). In any case, an excessively large amygdala volume is correlated with a greater tendency to anxiety, both in adults and children (in these excessively large amygdalae, there are more synapses, and it is the hyperconnectivity induced by these multiple synapses that generates anxiety). Thus, S Quin 2014 (Stanford University) who examined with fMRI, 76 children aged between 7 and 9 years old, observed an increase of the amygdala volume in anxious children. He also managed to establish that childhood anxiety is predictable based on the increase in volume of the left amygdala or of that of the left and right baso-lateral amygdaloid nuclei.
Equally interesting were the studies conducted on individuals with amygdalae with a volume below average, which are numerous and varied. Thus, correlations with an excessively small amygdala were developed by DA Pardini et al. 2014 who showed that the amygdaloid volume, which is smaller than average, in males, is associated with childhood aggressiveness, future violence and even psychotic features.
Post-traumatic stress syndromes were studied in particular: no amygdaloid changes were observed in adults from various backgrounds (FL Woon and DW Hedges 2008, 2009). The same authors noted no significant change in the volume of the amygdalae in children and adults who suffered from post-traumatic stress syndromes as a result of ill treatment during childhood. However, the study of post-traumatic stress syndromes among veterans revealed a decreased amygdaloid volume. The authors of this study (RA Morey et al. 2012) question whether this decrease in volume does not reflect the result but rather the vulnerability to this type of syndrome.
In people suffering from schizophrenia, there appears to be no significant reduction in the amygdaloid volume (SA Chance et al. 2002).
However, in bipolar psychotics, studied during their first episode, the volumes of the amygdala and cerebral white substance appear to be significantly reduced (IM Rosso et al. 2007).
SY Hill et al. 2013 also showed that a reduction in the amygdaloid volume is seen in the offspring of families with multiple alcohol-dependent members. Nevertheless, a study conducted by VV Senatorov et al. 2015 showed that alcoholics have an enlarged amygdala and reduced anterior insula.
The daily use of cannabis causes, over the long term, a reduction in volume of the hippocampus and of the amygdala (I Murat Yucel et al. 2008). We also know that a small amygdala increases the risk of addiction (N Makris et al. 2004).
Then there is the work of K Schiltz et al. 2007 who studied the brains of 15 male non-violent paedophiles and observed a size reduction of the right amygdala and a bilateral reduction in the hypothalamic grey substance of the septal region of the innominate substance and of the BST (anomalies unrelated to the age of the subjects being examined).
The specific case of autism has greatly interested neuroscientists who have looked for correlations between the size of the amygdalae of children who are affected and the intensity of their disorders (BM Nacewicz et al. 2006). In this puzzling disorder, where we can frequently observe increases in the amygdaloid volume, the most frequently explanation provided is that normal apoptosis phenomena, which normally occur in the amygdalae during the foetal period, do not take place or not sufficiently.
J Munson et al. 2006 focused more specifically on the volume increase of the right amygdala at the ages of 3 and 4 years. The greater the volume increase, the more the communication skills and social life will be disrupted.
CY Schumann et al. 2004 observed no difference in terms of total brain volume among a group of autistic children, aged between 7.5 and 12.5 years, and a control group, while the volumes of the left and right amygdalae of these autistic children were larger than those of the children belonging to the control group. However, in the group from 12.5 to 18.5 years old, the volume differences are no longer observed, which seems to show that the normal volume increase in standard amygdalae during adolescence does not occur in autistic children.
For JE Kim et al. 2010, children with abnormal social behaviours and communication problems have amygdalae whose nuclei of the baso-lateral group have an increased volume.
With the sequential images provided by MRI, we can obtain a relatively accurate idea of the orientation of the amygdala within the brain. On the axial sections, the large frontal axis of the amygdala is oriented slightly backwards and inwards. On the sagittal sections, we can observe that the large amygdalar axis is a little less oblique than that of the hippocampal head. On the frontal sections, the direction of the large axis of the amygdala shows that it is the result of the rotational movement undergone by this formation during its evolution. This axis, which was vertical, craniocaudal in primitive mammals, has been titled by 130–140, and in humans, it is directed obliquely downwards and outwards, as shown by the work of T Humphrey.
The special consistency of the cadaveric amygdala should also be noted. Its hardness contrasts with the fragility and soft, crumbly consistency of the peripheral cortex. This consistency is actually that of the constituent nuclei that appear dense, compact and crush resistant.
4.1.1 Subdivisions of the Amygdala: The Amygdaloid Nuclei
It is impossible to study these nuclei accurately by only using sections in various spatial planes. However, a well-conducted dissection, extracting the amygdala from its peripheral cortex envelope and then truly sculpting its surface, provides a good idea of the layout of these nuclei. Considering their hardness in anatomical subjects prepared by with formalin injections, such a dissection can generally be achieved in good conditions (Fig. 4.6).
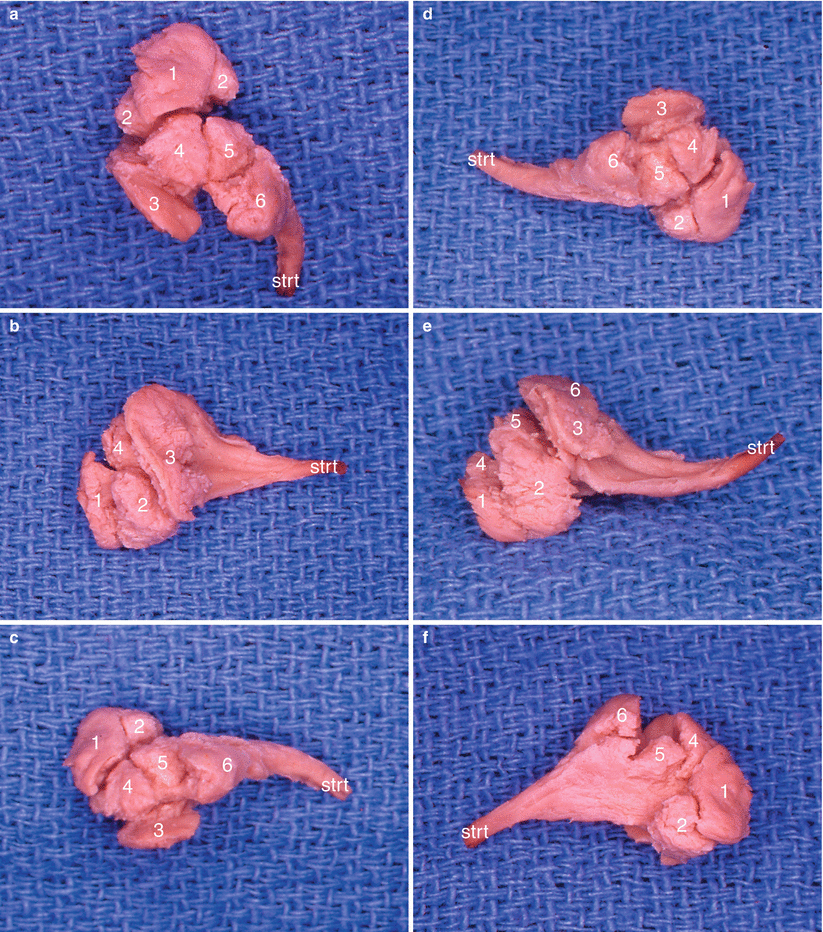

Fig. 4.6
Dissection of the right nuclear amygdaloid complex showing the arrangement of the nuclear subdivisions. 1 lateral nucleus, 2 baso-lateral nucleus, 3 baso-medial nucleus, 4 cortical nucleus, 5 central nucleus, 6 medial nucleus, strt stria terminalis. (a) antero-superior view; (b) antero-medial view; (c) superior view; (d) lateral view; (e) inferior view; (f) anterolateral view
4.1.1.1 Nuclei Forming the Amygdaloid Nuclear Complex
The authors only truly agree to affirm the heterogeneity of the amygdala, which is an assembly of cellular islands referred to as nuclei.
However, the number of constituent nuclei of the human amygdala varies according to the authors, the maximum being 30 nuclei for H Brockhaus 1938. Other authorsl such as EC Crosby and T Humphrey 1962 singularly reduced the segmentation of the human amygdala, not only by acknowledging a smaller number of nuclei but also by grouping these nuclei as superficial nuclei (medial and cortical), which are phylogenetically the oldest, and deep nuclei (lateral, basal and central), which are phylogenetically more recent.
Among the authors who have recently studied the nuclear arrangement of the human amygdala, we should specifically mention L Heimer et al. 1995, 2008, P Sah et al. 2003, JS De Olmos 2004 and CM Schuman and DG Amaral 2006. All of these authors found that each nucleus has specific characteristics not only morphological but also histochemical or cytoarchitectural. Qualitative differences (S Kemppainen et al. 2002) and quantitative differences (N Barger et al. 2012; CY Schumann and DG Amaral 2006) have thus been identified in the neuronal content of some amygdaloid nuclei in humans, and comparisons have been made with the amygdaloid nuclei of great apes. From the various studies, we can conclude that the evolution of the human amygdala did not consist of a simple increase in volume, at the same time as the brain volume increase, but rather of an adaptive reorganisation of the various constituent nuclei (K Semendeferi et al. 2010).
While EC Crosby and T Humphrey 1941, in their aim to summarise, had, as we saw, reduced the nuclei to only 2 groups, the cortico-medial group and the baso-lateral group, most authors acknowledge the current 4 anatomical groups which have different features:
The baso-lateral or deep group (complex) consists of three nuclei: the lateral, the baso-lateral and the baso-medial (or secondary baso-lateral or even secondary basal).
The cortical group or superficial group owes its name to the fact that due to its surface location, it is flush with the cortex, of which it adopts the layered structure. However, we have always found, during each dissection, a real cortical layer which covers these cortical nuclei. This layer is generally thin with respect to other parts of the cortex. Furthermore, several authors include in this cortical group the entire periamygdaloid cortex that they consider as equivalent to a nucleus. The other nuclei belonging to this group are the anterior cortical and posterior cortical nuclei, the nucleus of the lateral olfactory tract and that of the secondary olfactory tract, which amounts to 5 nuclei for the superficial group.
The centro-medial group has 3 nuclei, among which 2 are essential from a functional viewpoint: the central nucleus and the medial nucleus. The third nucleus corresponds to the amygdaloid part of the nucleus of the bed of stria terminalis.
The group of additional nuclei includes the anterior amygdaloid area (visible on axial and sagittal sections), the amygdalo-hippocampal area and the amygdala-piriform area (visible on the coronal sections) and smaller clusters of neurons called intercalated nuclei because they are located between the large nuclei that we have just listed and which are as the latter, cellular islands limited by a fibrillar envelope.
4.1.1.2 Nomenclature of the Nuclei
According to us, the nomenclature defined by JS De Olmos 2004 seems to be the most appropriate. De Olmos has the great merit of naming each of the nuclei by abbreviations but also of reviewing and clarifying the amygdaloid nomenclature.
We therefore have: in the latero-basal group, the lateral (La), the baso-lateral (BL) and the baso-medial (BM); in the cortico-medial group, the cortical (Co); and in the centro-medial group, the central (Ce) and the medial (Me).
Each nucleus has subsequently been organised into subdivisions that may confuse novices but which provide a high level of accuracy and a fully functional labelling system (Fig. 4.7).
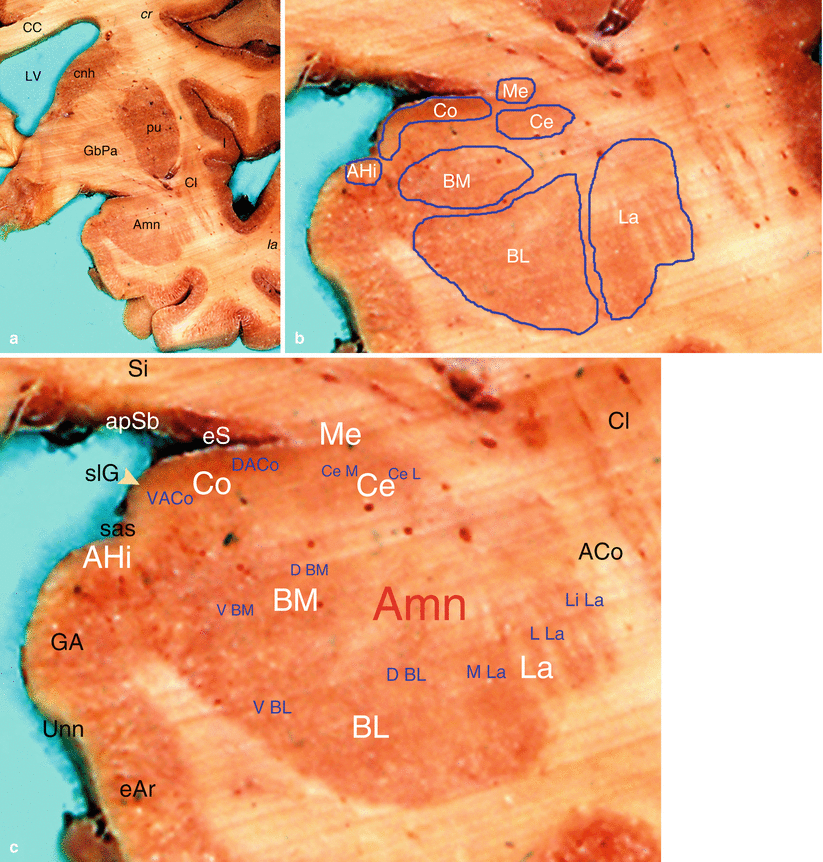

Fig. 4.7
The nuclei of the human amygdala. (a) The left amygdala in situ on a coronal section of the brain (cr, cranial; la, lateral). (b) Manual drawing of the amygdala’s nuclei coloured by FeCl3. (c) An overview of the nuclei according to De Olmos’s classification. ACo anterior commissure, Amn amygdaloid nuclear complex (AHI amygdalo-hippocampal area, BL baso-lateral nucleus, D BL dorsal subdivision sd, of BL, V BL ventral sd of BL, BM baso-medial n, D BM dorsal sd of BM, V BM ventral sd of BM, Ce central nucleus, Ce L lateral sd of Ce, Ce M medial sd of BM, Co cortical n, DA Co dorsal anterior sd of Co, VA Co ventral anterior sd of Co, La lateral n, Li La limitans sd of LA, L LA lateral sd of LA, M La medial sd of LA, Me medial n), apSb anterior perforated substance, CC corpus callosum, Cl claustrum, cnh caudate nucleus head, eAr entorhinal area, eS entorhinal sulcus, GA gyrus ambiens, GbPa globus pallidus, I insula, LV lateral ventricle, pu putamen, sas semi-annular sulcus, slG semilunar gyrus, Si substantia innominata, Unn uncal notch
The lateral nucleus, which obviously comprises a lateral portion and a medial portion, was divided into L La and M La divisions. Each lateral and medial division comprises a dorsal portion and a ventral portion, separated by an intermediate division I La. There is also a limiting division labelled Li La.
The baso-lateral nucleus has been divided into a dorsal portion D BL and a ventral portion V BL, separated by an intermediate portion I BL.
The baso-medial nucleus has been divided into 4 portions, the dorsolateral portion DL BM, the dorsomedial portion DM BM, the ventrolateral portion VL BM and the ventromedial portion VM BM.
The central nucleus has been divided into a lateral portion Ce L and a medial portion Ce M, the latter being divided itself into a dorsal portion D Ce M and a ventral portion V Ce M.
The medial nucleus has been divided into an anterior portion Me A and a posterior portion Me P, which itself has been divided into a dorsal portion D Me P and a ventral portion V Me P.
It is the cortical nucleus that poses the most problems. Co comprises three parts:
An anterior nucleus ACo with a dorsal division D ACo and a ventral division V ACo
A ventral nucleus VCo with a caudal division C VCo and a rostral division R VCo.
R VCO itself is divided into an inferior portion IF R VCo, a superior portion Sp R VCo and an intermediate portion IT R VCo.
As for the anterior nucleus, labelled anterior amygdaloid area AAA, it is divided into two subdivisions, the first one, the deep, Dp AAA, deep division of AAA, and the other, superficial, Sf AAA, superficial division of AAA.
Two junction areas surround the cortical nucleus:
The amygdalo-piriform area APir, including an anterolateral division AL APir and a postero-medial division PM APir
The amygdalo-hippocampal area AHi which includes an anterolateral division AL AHi and a postero-medial division PM AHi
We should also mention the amygdalo-striatal transitional area AStr and the amygdalo-claustral area ACA included in this nomenclature by the author’s highly accurate mind.
4.1.1.3 Highlighting the Nuclei
The nuclei are perfectly visible on histological sections after Nissl staining. Using more sophisticated staining techniques, such as silver plating, is not required.
Our surface staining of serial brain sections with FeCl32 is also a great way of enhancing the visualisation of the amygdaloid nuclei, even if the amygdala is coloured with less intensity than other central grey nuclei. It is above all a simple and inexpensive technique which can be used extemporaneously by a neuroanatomist (Figs 4.8, 4.9, 4.10, 4.11, 4.12, 4.13 and 4.14).
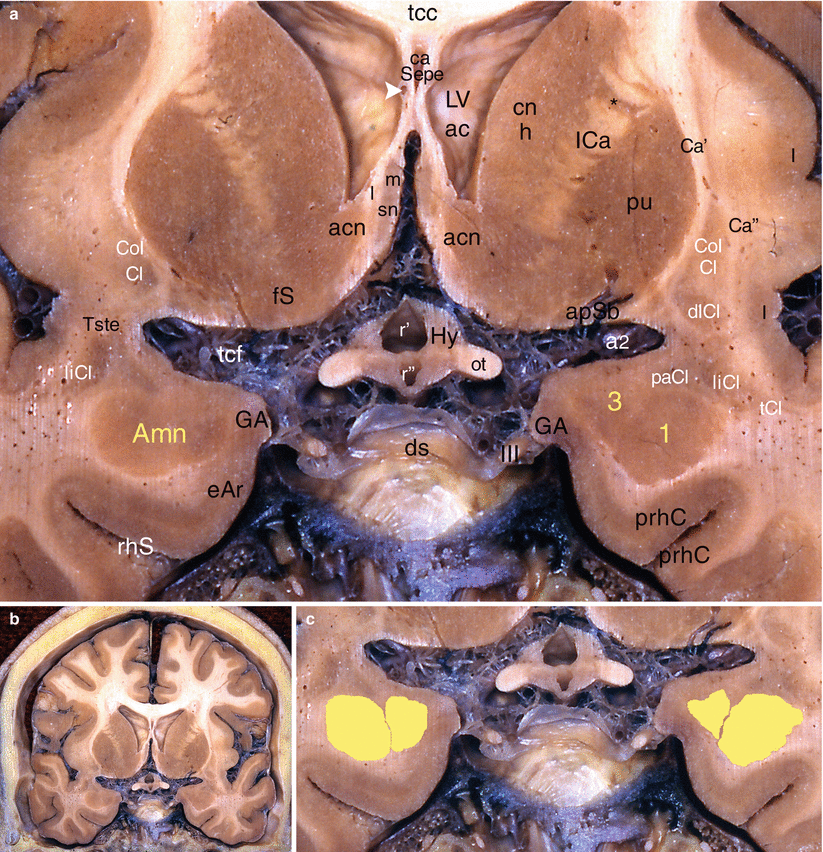
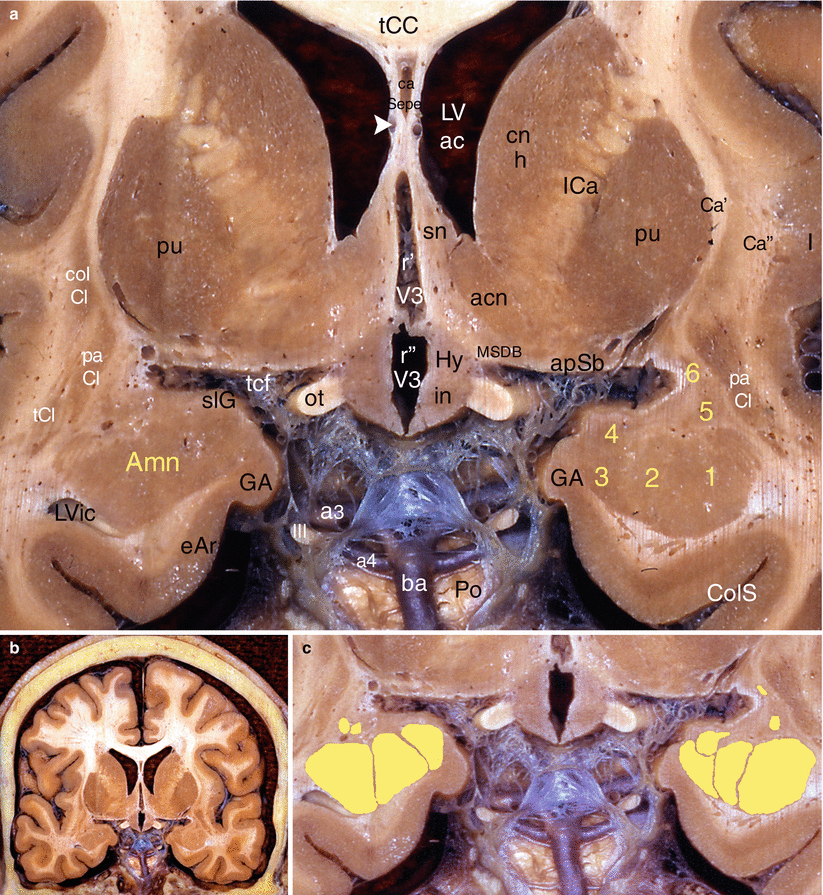
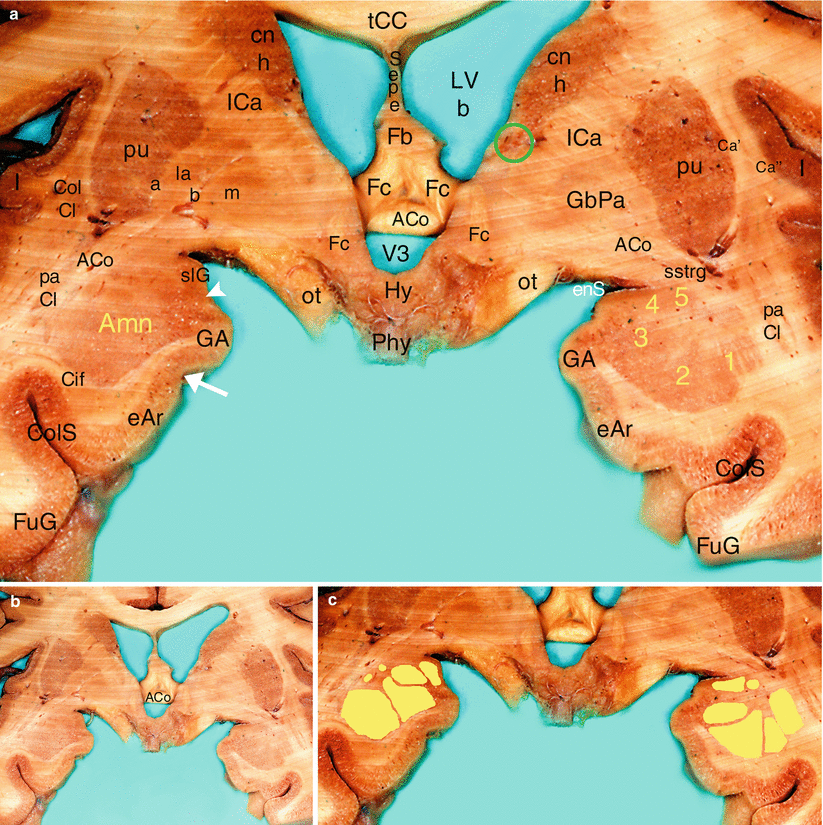
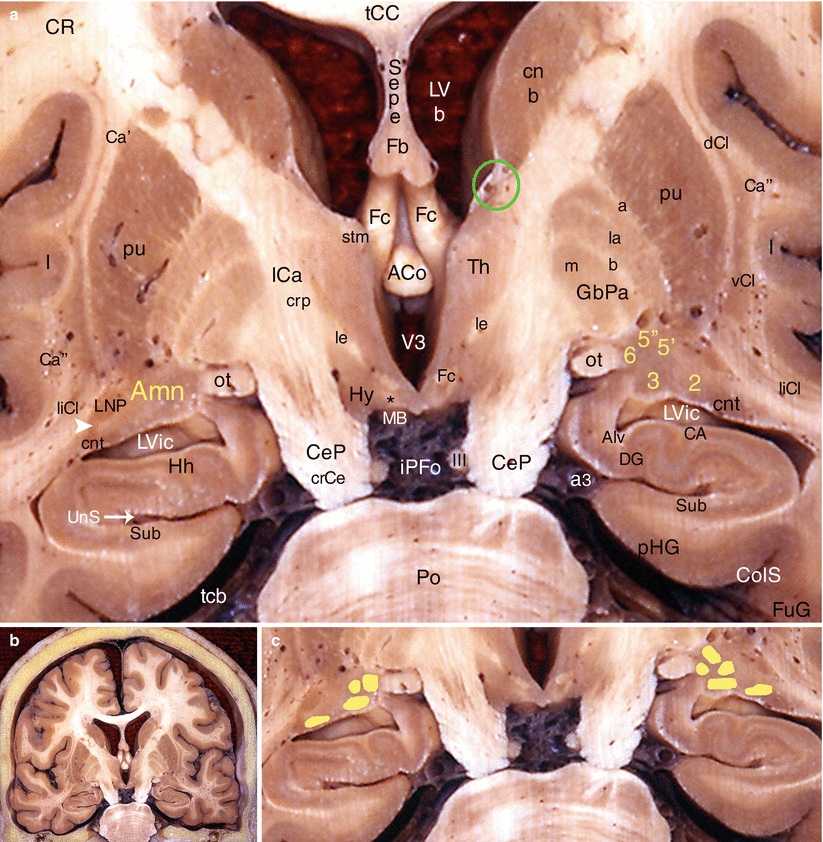
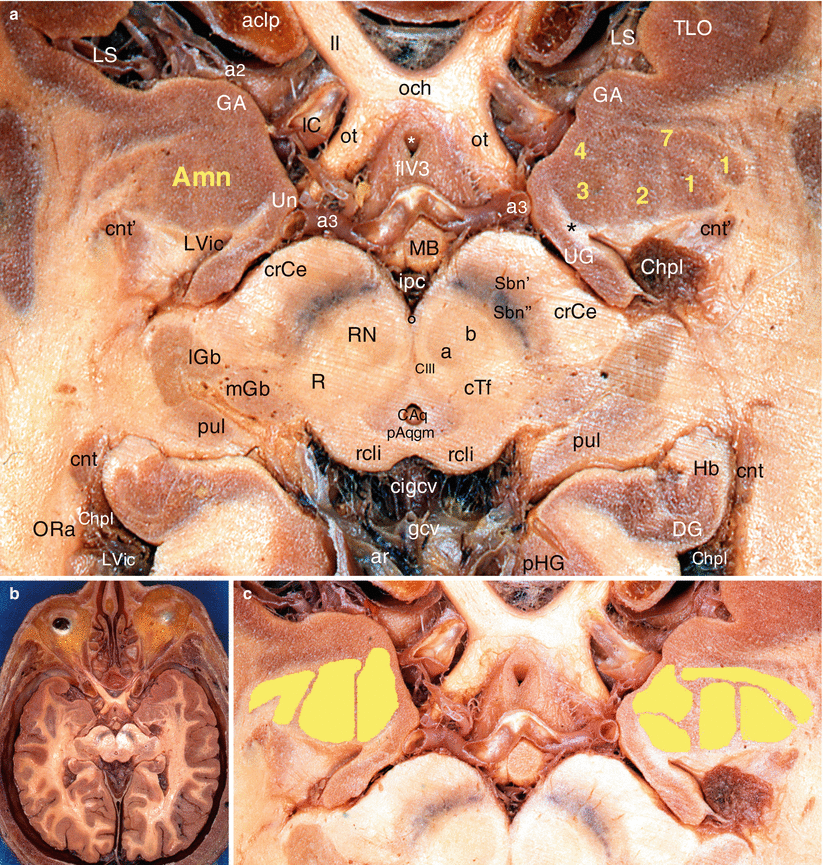

Fig. 4.8
The amygdaloid nuclear complex on a coronal section of the brain through the septal area. (a) Details, (b) general situation, (c) nuclear organisation of Amn. acn accumbens nucleus, Amn amygdaloid nuclear complex, 1 lateral nucleus, 3 baso-medial nucleus, apSb anterior perforated substance, Ca’ external capsule, Ca” extreme capsule, ca Sepe cavity of the septum pellucidum, cn h caudatus nucleus (head), CoICl compact insular claustrum, dICl diffuse insular claustrum, ds dorsum sellae (sphenoidal bone), eAr entorhinal area, fS fundus striati, GA gyrus ambiens, Hy hypothalamus, I insula, ICa internal capsule, liCl limitans claustrum, LVac lateral ventricle (anterior cornu), ot optic tract, paCl pre-amygdalar claustrum, prhC perirhinal cortex, pu putamen, r’ optic recess of V3, r” infundibular recess of V3, rhS rhinal sulcus, sn septal nuclei, m medial, l lateral, tCC truncus of the corpus callosum, tcf transverse cerebral fissure, tCl temporal claustrum, Tste temporal stem, III oculomotor nerve, a2 middle cerebral artery, white arrowhead venae of the septum pellucidum, black asterisk caudato-lenticular grey cell bridge

Fig. 4.9
The amygdaloid nuclear complex on a coronal section of the brain through the rostral part of V3. (a) Details, (b) general situation, (c) nuclear organisation of Amn. acn accumbens nucleus, Amn amygdaloid nuclear complex, 1 lateral nucleus, 2 baso-lateral nucleus, 3 baso-medial nucleus, 4 cortical nucleus, 5 central nucleus, 6 medial nucleus, apSb anterior perforated substance, ba basilar artery, Ca’ external capsule, Ca” extreme capsule, ca Sepe cavity of the septum pellucidum, cn h caudatus nucleus (head), coICl compact insular claustrum, ColS collateral sulcus, eAr entorhinal area, GA gyrus ambiens, Hy hypothalamus, I insula, ICa internal capsule, in infundibular nucleus, LVac lateral ventricle (anterior cornu), LVic lateral ventricle (inferior cornu), MSDB medial septum-diagonal band, ot optic tract, Po pons, paCl pre-amygdalar claustrum, pu putamen, slG semilunar gyrus, sn septal nuclei, tCC truncus of the corpus callosum, tcf transverse cerebral fissure, tCl temporal claustrum,V3 third ventricle, r’ optic recess of V3, r’’ infundibular recess of V3, III oculomotor nerve, a3 posterior cerebral artery, a4 superior cerebellar artery, white arrowhead venae of the septum pellucidum

Fig. 4.10
The Amygdaloid nuclear complex on a coronal section of the brain through the anterior commissure. (a) details (b) general situation (c) nuclear organisation of Amn. ACo anterior commissure, Amn amygdaloid nuclear complex, 1 lateral nucleus, 2 baso-latéral nucleus, 3 baso-medial nucleus, 4 cortical nucleus, 5 central nucleus, Ca’ external capsule, Ca” extreme capsule, Cif cingulate fasciculus, cn h caudatus nucleus head, CoICl compact insular claustrum, ColS collateral sulcus, eAr entorhinal area, enS endorhinal sulcus, Fb body of the fornix, Fc column of the fornix, FuG fusiform gyrus, GA gyrus ambiens, GbPa globus pallidus, la pars lateralis, m pars medialis, a external medullary lamina, b internal medullary lamina, Hy hypothalamus, I insula, ICa internal capsule, LVb lateral ventricle body, ot optic tract, paCl pre-amygdalar claustrum, Phy peduncle of the hypophysis, pu putamen, Sepe septum pellucidum, slG semilunar gyrus, sstrg substriatal grey, tCC truncus of the corpus callosum, V3 third ventricle, green circle BNST, white arrowhead semi-annular sulcus, white arrow uncal notch

Fig. 4.11
The amygdaloid nuclear complex on a coronal section of the brain through the cerebral peduncles and the pons behind the columns of the fornix. (a) Details, (b) general situation, (c) nuclear organisation of Amn. ACo anterior commissure, Alv alveus, Amn amygdaloid nuclear complex, 2 baso-lateral nucleus, 3 baso-medial nucleus, 5’ central nucleus (lateral part), 5” central nucleus (medial part), 6 medial nucleus, CA cornu ammonis, Ca’ external capsule, Ca” extreme capsule, CeP (crCe) cerebral peduncle (crus cerebri), cnb body of the caudatus nucleus, cnt tail of the caudatus nucleus, CR corona radiata, ColS collateral sulcus, dCl dorsal claustrum, DG dentate gyrus, Fb body of the fornix, Fc column of the fornix, FuG fusiform gyrus, GbPa globus pallidus, la pars lateralis, m pars medialis, a external medullary lamina, b internal medullary lamina, Hh hippocampus head (digitationes hippocampi, Hy hypothalamus, I insula, ICa (crp): internal capsule (crus posterior), iPFo interpeduncular fossa, le fasciculus lenticularis (field H2 of Forel), liCl limitans claustrum, LNP lentiform nucleus peduncle, LVb body of the lateral ventricle, LVic inferior cornu of the lateral ventricle, MB mamillary body, ot optic tract, pHG parahippocampal gyrus, Po pons, pu putamen, Sepe septum pellucidum, stm stria medullaris thalami, Sub subiculum, tcb tentorium cerebelli, tCC truncus of the corpus callosum, Th thalamus, UnS (white arrow) uncal sulcus V3: third ventricle, vCl ventral claustrum, III oculomotor nerve, a3 posterior cerebral artery, black asterisk mamillothalamic fasciculus, green circle NBST, NB notice the grey bridge cell (white arrowhead) between the tail of the caudatus nucleus and LNP

Fig. 4.12




The amygdaloid nuclear complex on an axial section of the brain through the mesencephalon (midbrain) and the optic chiasm. (a) Details, (b) general situation, (c) nuclear organisation of Amn. Amn amygdaloid nuclear complex, 1 lateral nucleus, 2 baso-lateral nucleus, 3 baso-medial nucleus, 4 cortical nucleus, 7 anterior nucleus, aclp anterior clinoid process, ar arachnoid mater, CIII oculomotor nerve’s nuclear complex, Caq cerebral aqueduct, Chpl choroid plexus, cigcv cisterna of the great cerebral vein, cnt caudate nucleus tail, cnt’ para-amygdalar part of the caudate nucleus tail, crCe crus cerebri, cTf central tegmental fasciculus, DG dentate gyrus, flV3 floor of V3, GA gyrus ambiens, gcv great cerebral vein, Hb hippocampus body, IC internal carotid, ipc interpeduncular cisterna, lGb lateral geniculate body, LS lateral sulcus, LVic lateral ventricle, inferior cornu (temporal horn), MB mamillary body, mGb medial geniculate body, och optic chiasm, ORa optic radiations, ot optic tract, pAqgm periaqueductal grey matter, pHG parahippocampal gyrus, pul pulvinar, R reticular formation, rcli rostral colliculus, RN red nucleus, a magnocellular part, b parvocellular part, Sbn’ substantia nigra, reticular part, Sbn” substantia nigra, compact part, TLO temporal lobe, UG uncinate gyrus, Un uncus, a2 middle cerebral artery, a3 posterior cerebral artery, II optic nerve, black asterisk cingulate fasciculus(Cif), white asterisk infundibular recess (inr), black circle interpeduncular nucleus
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree