and Alfredo Blandino1
(1)
Department of Radiological Sciences, University of Messina, Messina, Italy
Inflammatory bowel disease (IBD) primarily refers to two chronic diseases that cause chronic inflammation of all or part of the digestive tract: ulcerative colitis (UC) and Crohn’s disease (CD).
UC predominantly affects the colon, with the exception of “backwash ileitis,” which involves the terminal ileum. In such cases, colonoscopy is therefore the most effective method to assess the activity of the disease.
CD is an idiopathic, chronic, transmural, inflammatory disease that can affect any part of the gastrointestinal tract from mouth to anus, with a tendency toward segmental distribution and often involving multiple discontinuous sites (skip lesions). Most commonly, it affects the small bowel, which is (apart from the terminal ileum) usually not accessible with conventional endoscopic techniques.
In about 70–80 % of patients with CD, there is small bowel involvement, and in about 20–30 %, the disease is limited to the small bowel. The colon can be affected either with (50 % of cases) or without (15–20 %) the involvement of the small intestine [1].
The incidence of CD seems to have a bimodal distribution, the first peak occurring in late adolescence and early adulthood while a second smaller increase in incidence can be seen between the fifth and seventh decade of life [2, 3]. Prevalence in many developed countries is estimated at 0.1 % [4]. A familial tendency has also been described, observing an increased risk of ulcerative colitis in relatives [5].
The etiology and pathogenesis of CD are not completely understood, but there is increasing evidence that genetic as well as environmental factors may play an important role in causing a sustained activation of mucosal immune responses, which lead to cytokine overproduction and subsequently to leukocytary infiltration of the bowel wall [2, 6, 7].
Furthermore, luminal bacteria and microbiota would also likely play a major role in the pathogenesis of CD.
Microscopically, the initial lesion starts as a focal inflammatory infiltrate in the mucosa and submucosa, which leads to hyperemia and edema.
Macroscopically, the earliest feature of CD is a shallow aphthoid mucosal ulceration, histologically corresponding to initial mucosal ulceration over a mucosal lymphoid follicle. As the disease progresses, aphthoid ulcers develop and further progress into extensive linear ulcers and fissures. Advanced ulcerations with bulging of the edematous residual mucosal islands produce the typical ulceronodular or “cobblestone” appearance. In severe cases, transmural inflammation and serosal involvement are present as well. The bowel wall can also result to be thickened by a combination of fibrosis and inflammatory infiltrates. In long-standing cases, chronic obstruction due to scarring, luminal narrowing, and stricture formation may arise.
For what concerns extramural manifestations, these are fistulas, abscesses, adhesions, creeping fat, and enlargement of the lymph nodes.
Although it is not common, toxic megacolon and neoplasms such as lymphoma and carcinoma may also occur [8].
Symptoms of CD are often unpleasant and they are classically represented by abdominal pain, weight loss, diarrhea (which may include blood or mucus and may vary in frequency), and tenesmus; however, it can have different presentations and tends to have an unpredictable course marked by flares, remissions, and relapses.
The diagnosis of CD is based on a combination of clinical findings, endoscopic appearance, biopsy, radiological studies, and biochemical markers; however, most commonly, ileocolonoscopy and biopsies from the terminal ileum and colon are the ones used to determine the initial diagnosis.
With regard to the imaging of CD at initial presentation and in the setting of clinical suspicion, as previously stated in the introduction, MR of the small bowel can be considered a useful support both in patients with an incomplete ileocolonoscopy and in those with negative ileocolonoscopy, to, respectively, exclude enteric inflammation at the level of the terminal ileum or proximal to this site.
MR of the small bowel can also be used as a first-line diagnostic approach in pediatric patients with clinical suspicion for CD, or it can be performed prior to video capsule endoscopy (very sensitive to detect subtle mucosal disease), to exclude strictures.
However, in the majority of cases, MREg/MREc is primarily employed to assess the exact location and extent of inflammatory bowel disease in patients with histological diagnosis of CD.
It should also be considered that in clinical practice, CD is stratified by disease severity (mild, moderate, and severe), disease location (upper gastrointestinal, ileal, ileocolonic, colonic, or perianal), extent of disease, and disease phenotype (penetrating, structuring, or inflammatory – nonpenetrating nonstricturing disease) [9].
MR helps to categorize the relative components of inflammatory, penetrating, or structuring disease in each individual patient, and it should be used for the surveillance of patients with known CD.
The assessment of the inflammatory activity is essential in order to delineate treatment strategies and determine the optimal choice and dose of medication; at the same time, the monitoring of patient is important to evaluate the efficacy of treatment [10].
In addition to dietary approach, several medical therapies are used to induce and/or maintain remission in CD, including corticosteroids, immunomodulators (e.g., azathioprine, mercaptopurine, methotrexate), and biologic agents (e.g., TNF-alpha inhibitors). In particular, with the advent of new medication such as infliximab, an immunomodulatory drug with considerable side effects, follow-up to determine therapy efficacy is becoming increasingly important.
Several clinical scoring systems have been developed to assess disease activity and response to therapy, the most common of which is the Crohn’s Disease Activity Index (CDAI), although it is far from being a conclusive metric to evaluate the severity of the disease.
Moreover, identification of location of fibrotic restricted lumen tracts and evaluation of prestenotic dilatation may help surgeons to plan intervention [10]. However, surgery is delayed until the disease does not respond to pharmacotherapy or there are complications, such as obstruction. When performed, bowel conservation surgery is the aim.
In this context, MRI can demonstrate active small bowel inflammation recognizing wall thickening, ulcerations, increased wall enhancement, increased vascularity, perienteric inflammation, and reactive adenopathy. MR also allows more accurate identification of associated complications including penetrating and fibrostenotic disease as well as the more rare extraintestinal manifestations that are usually associated with severe and long-standing intestinal inflammation, the latter often guiding the therapeutic approach [11–16].
4.1 Wall Thickening
Bowel wall thickening is the most reported findings of CD even if not entirely specific.
In adequately distended small bowel, the normal wall thickness is 1–3 mm, while a bowel wall thickening more than 3 mm can be regarded as abnormal [17].
It has also be shown that mural thickness increases with acute inflammation, generally ranging between 5 and 10 mm (given the associated histological findings of edema and inflammatory infiltrate) and that, in patients with CD, this well correlates with the presence and severity of the disease [18]. An associated thickening of the mucosal folds can be also seen (Fig. 4.1).
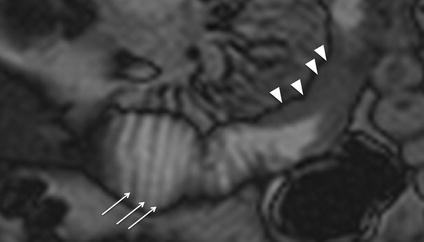

Fig. 4.1
Wall thickening. Magnified coronal TrueFISP image shows wall thickening of a proximal ileal tract (arrowheads) and thickening of mucosal folds in the segment next to it (arrows)
Although the thickness decreases during remission, the inactive but yet pathological bowel is likely to be thicker compared with the completely normal bowel [18, 19]. To help the radiologist in distinguishing between active and inactive disease, a cut-off thickness of 6 mm has been proposed [19].
The most common site of CD is the terminal ileum (Fig. 4.2), sometimes with contiguous disease in the cecum. Discontinuous skip lesions may also be seen more proximally in the small bowel (Fig. 4.3) or within the colon.
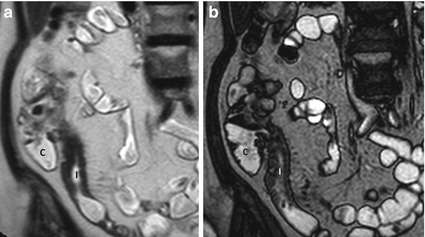
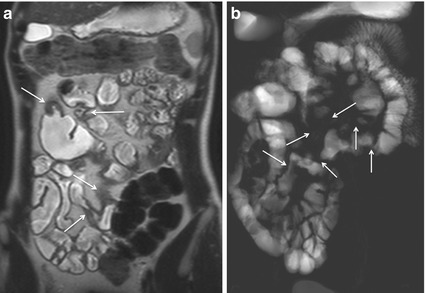

Fig. 4.2
Wall thickening. Magnified coronal HASTE (a) and TrueFISP (b) images show wall thickening of the terminal ileum (I). C cecum

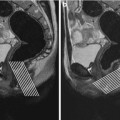
Fig. 4.3
Skip lesions. Coronal HASTE (a) and coronal MR-fluoroscopy images (b) depict multiple inflammatory strictures (arrows) separated by segments of both normally distended and dilated small bowel segments
The involvement of the bowel wall may be symmetrical or asymmetrical, where greater involvement of the mesenteric border can lead to pseudosacculation. Fibrosis and shortening of the diseased mesenteric wall lead to apparent dilatation of the opposing normal bowel wall (Fig. 4.4). Because all three bowel wall layers form the sacculation (in contrast to colonic diverticular disease), such a finding may also be referred to as a pseudodiverticulum [20].
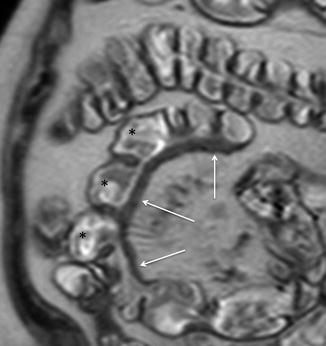

Fig. 4.4
Asymmetrical wall thickening and pseudosacculations. Magnified coronal HASTE image shows multiple pseudosacculations on the antimesenteric border (asterisks) due to asymmetric thickening of the bowel wall (arrows)
Moreover, if there is suboptimal distention (as sometimes is the case, especially with jejunal loops), the disease may be overestimated or underestimated [17]. The evaluation of all images, sequentially acquired, may help to clarify the extent of bowel involvement, as fluid distension in the small bowel will vary over time.
Mural thickening can be appreciated on all sequences. However, while thickness can be assessed with both T1- and T2-weighted sequences, balanced SSFP sequences should be avoided due to the “black border artifact,” which can complicate the evaluation of bowel wall thickness. Conversely, HASTE sequence, which is insensitive to chemical shift and is not susceptible to black border artifact, will be indicated for more accurate measurements [16, 17].
Fat-saturated T2-weighted sequences are very helpful for characterizing the etiology of wall thickening, appearing as an area of high signal intensity in the presence of active inflammation, due to the edema (Figs. 4.5 and 4.6); this finding has been shown to well correlate with the severity of inflammation, and it can be particularly useful in patients with contraindications to contrast media administration [16].
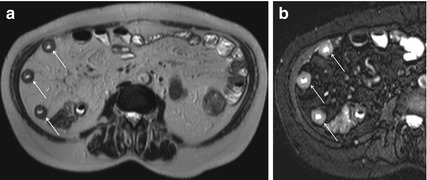
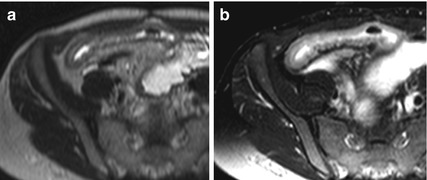

Fig. 4.5
Wall thickening in active Crohn’s disease. Axial HASTE image (a) showing concentric bowel wall thickening in multiple segments (arrows). On magnified axial HASTE fat-saturated image (b), obtained at the same level, it is demonstrable high signal intensity of the diseased intestinal tracts, as a consistent finding of inflammatory edema in active Crohn’s disease

Fig. 4.6
Wall thickening in active Crohn’s disease. Magnified axial HASTE image showing concentric wall thickening of the distal ileum (a). Magnified axial HASTE fat-saturated image, obtained at the same level (b), depicts high signal intensity of the diseased intestinal segment, as a consequence of inflammatory edema, a consistent finding of active Crohn’s disease
In addition, in severe CD, the inflamed bowel wall has a layered appearance on T2-weighted sequences with a high signal intensity ring, representing the submucosal edema, between the mucosa and serosa (“target sign”) [16, 21]. The hyperintensity of the submucosa also persists in the fat-saturated T2 images (Fig. 4.7).
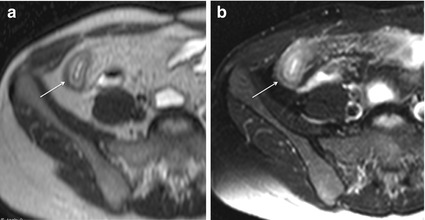

Fig. 4.7
“Target sign” in severe active Crohn’s disease. Axial HASTE images in a patient with severe active Crohn’s disease show a layered appearance of the inflamed terminal ileum (arrow), due to hyperintense submucosal edema (a), still visible after fat saturation (b)
Because on HASTE sequence, intramural fat deposition in chronic disease may result in elevated T2 signal intensity, fat-saturated T2-weighted sequences are also recommended to differentiate edema from fat: loss of signal on T2-weighted fat-saturated sequences is suggestive of chronic disease and this can be a helpful feature in interpreting the significance of thickened bowel wall [16, 21]. In these cases, a target appearance may be produced by low signal intensity “ring or halo” due to fat hypertrophy and fibrosis of the submucosa (“fat halo sign”) (Fig. 4.8).
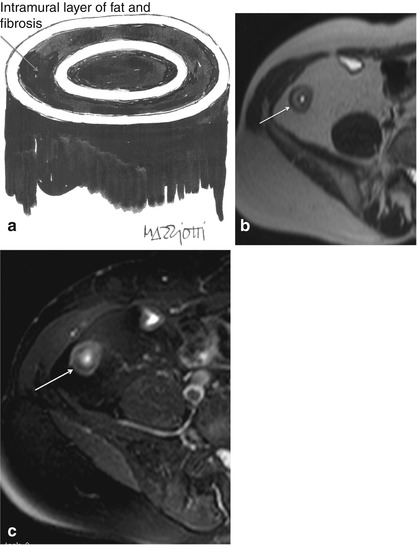

Fig 4.8
“Halo sign” in chronic Crohn’s disease. Illustration showing the “halo sign” (a), caused by fat hypertrophy and fibrosis of the intestinal submucosa in chronic Crohn’s disease. Axial HASTE image (b) shows a layered appearance of the terminal ileum (arrow), which is due to hyperintense submucosal fibrofatty proliferation, as confirmed by the lack of signal in the magnified HASTE fat-saturated image (c) (Note differences with active Crohn’s disease, seen previously in Fig. 4.7)
Recently, DWI, along with associated ADC measurements, has been shown to reflect abnormal activity in CD patients, helping to further characterize the bowel wall thickening and complementing the morphological information obtained by conventional MRI [22–24].
Particularly, active inflammatory thickening of the bowel wall is characterized by brighter signal in the b-value images and lower ADC values relative to normal segments (Fig. 4.9).
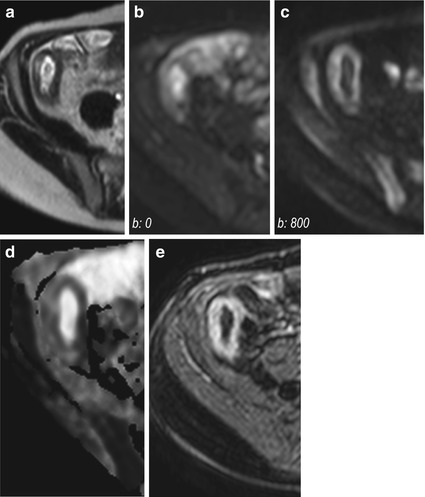

Fig. 4.9
Active Crohn’s disease. Magnified axial HASTE image (a) shows wall thickening of the terminal ileum. Axial DW images performed at different b-values (b: 0 s/mm2 and 800 s/mm2, respectively) show persistent hyperintense signal of the pathologically thickened wall of the terminal ileum (b, c). The ADC(0–800) map (d) demonstrates restricted diffusion (dark region) in the site of abnormal terminal ileum, which well correlates with marked hyperenhancement on axial fat-saturated T1-weighted image obtained after i.v. administration of gadolinium (e)
The visual assessment of DWI may provide higher accuracy, while calculation of the ADC may facilitate the quantitative analysis of the activity of the disease [22–24].
In the presence of a borderline thickening of the small bowel wall, dynamic cine-MR sequences can more accurately demonstrate the number of pathological intestinal segments than what static MRI can do. In fact, abnormally decreased or increased peristalsis may be an early sign of the bowel involvement by CD and can potentially help to identify affected segments that would appear with only subtle or doubtful signs of inflammation on static images. However, it should be well kept in mind that additional factors over and above acute inflammation might affect motility in CD, including drug regimens, disease duration, location, coexistence of colitis, previous surgery, and mural fibrosis [25–28].
4.2 Ulcerations
The microscopic examination of the intestinal mucosa in patients affected by CD shows active inflammation, which is usually characterized by varying degrees of injury of the neutrophilic crypts.
In mild CD, only a small fraction of crypts is infiltrated by neutrophils; crypt destruction and mucin depletion can also be seen. As the degree of activity increases, there is a corresponding escalation of the involved crypts and of the severity of crypt injury, which includes the necrosis of epithelial crypts, crypts abscess, and the eventual formation of ulcers.
Two types of ulcers can be seen in CD: the superficial aphthoid ulcers and the deep fissuring ulcers, more problematic than superficial ones.
A good depiction of ulcerations is highly dependent on the quality of luminal distention.
The deep fissuring ulcers usually break through the mucosa and into the deeper layers of the bowel wall, causing initially submucosal inflammation and edema [15, 29, 30].
However, when the ulceration is initial and superficial, it is not always well demonstrable at MRI, even if full luminal distention was achieved; on the other hand, conventional fluoroscopy, if well performed, still holds this advantage.
Fissuring ulcers primarily manifest as areas of erosion in the mucosal lining, and they may finally extend into the submucosal space, damaging the mucosal lining [15, 17, 29, 30].
Larger deep ulcers are outlined by luminal contrast material and appear as thin lines of high signal intensity longitudinally or transversely oriented into the thickened bowel wall (Fig. 4.10).
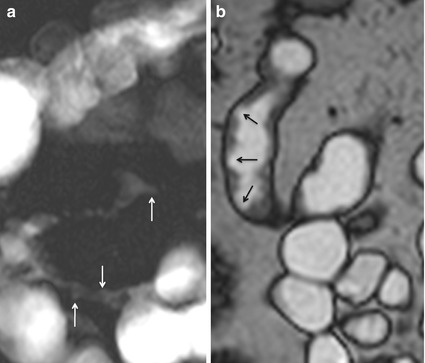

Fig. 4.10
Ulcerations. Magnified coronal MR fluoroscopy (a) shows ileal narrowing and ulcerations (arrows). Coronal high-resolution TrueFISP image (b) clearly depicts ulcerations as well (arrows)
HASTE and TrueFISP images, which provide high contrast between the lumen and the bowel wall, may facilitate the detection of transmural ulcers [29].
The cobblestone aspect, which results from multiple confluent and intersecting longitudinal and transverse ulcerations, with residual mucosal islands, appears as sharply demarcated and patchy areas of high signal intensity, a suggestive finding of advanced disease that may prompt a change in medical treatment (Fig. 4.11) [16, 29].
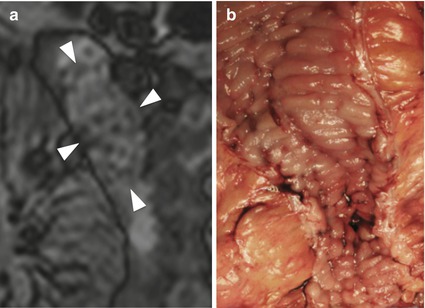

Fig. 4.11
“Cobblestone appearance” in Crohn’s disease. Magnified coronal high-resolution TrueFISP image (a) demonstrating the cobblestone appearance of edematous mucosa between longitudinal and transverse linear ulcers (arrowheads). Note the good correlation with the endoscopic findings (b)
4.3 Increased Vascularity
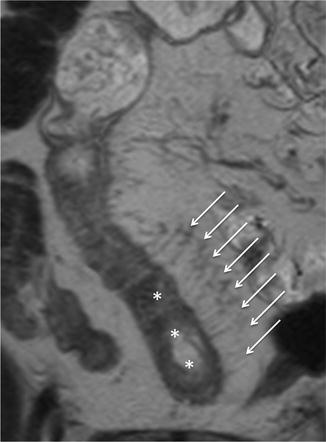
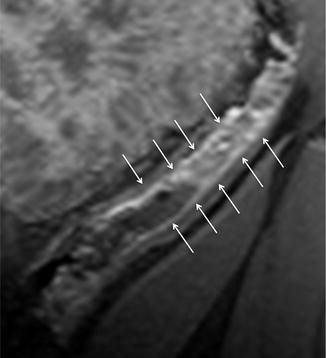
The increased mesenteric vascularity adjacent to inflamed bowel loops is often present in the setting of acute inflammation. In fact, when mesenteric blood flow increases, it results in engorgement of the vasa recta supplying the inflamed bowel segments, which appear as multiple tubular, tortuous opacities on the mesenteric side of the ileum, aligned like the teeth of a comb (“comb sign”) [16, 17, 29].
This finding, due to contrast enhancement of the vasculature, is frequently best seen as high signal intensity parallel lines on contrast-enhanced T1-weighted fat-suppressed images (Fig. 4.12). Moreover, it can also be seen on fat-suppressed HASTE or TrueFISP images, respectively, as high and low signal intensity parallel lines (Figs. 4.13 and 4.14).
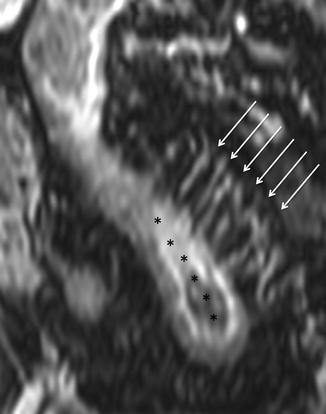
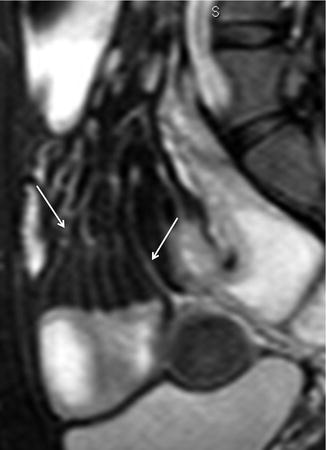
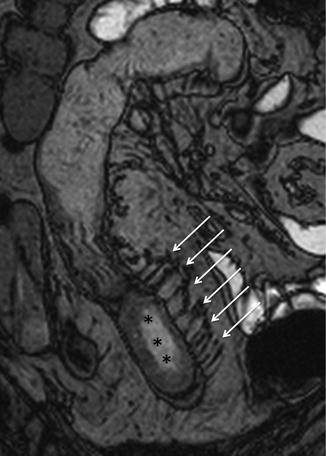

Fig. 4.12
“Comb sign” in Crohn’s disease. Coronal GE T1-weighted and fat-saturated image, obtained after i.v. injection of gadolinium. Ectatic vasa recta (arrows) supplying a diseased bowel loop (asterisks)

Fig. 4.13
“Comb sign” Crohn’s disease. Sagittal HASTE fat-saturated image showing ectatic vasa recta as hyperintense linear structures (arrows), directed from mesentery toward a diseased bowel segment

Fig. 4.14
“Comb sign” in Crohn’s disease. Same patient of Fig. 4.12. Coronal TrueFISP image showing ectatic vasa recta (arrows), represented by multiple linear hypointensities directed toward the pathological small bowel tract (asterisks)
The identification of comb sign, however, is much more limited on the nonfat-suppressed HASTE, due to the poor contrast resolution between vessels and mesenteric fat (Fig. 4.15).
4.4 Patterns of Wall Enhancement
Bowel wall enhancement plays a very important role in determining disease severity, as it can be one of the earliest signs of inflammatory activity.
It has been frequently reported to correlate with bowel inflammation and activity of the disease.
In CD, in fact, a marked increase in signal intensity of the inflamed bowel wall can be seen after intravenous gadolinium administration, due to increased tissue perfusion and vascular permeability.
The enhancement pattern of the inflamed bowel has also been studied to assess inflammatory activity as a comparison with clinical indices, and a significant enhancement decrease has already been correlated to good response to medical treatment [17, 18].
The assessment of enhancement should be done during several phases, based on the different scanning times, relatively to contrast injection.
Four enhancement patterns can be described and may help the gastrointestinal radiologist to determine the likely level of inflammatory activity:
1.
Get Clinical Tree app for offline access

Pattern I – mucosal hyperenhancement may be the first sign of active inflammation, even in the absence of a substantial wall thickening (Fig. 4.16).