A diagnosis of localized prostate cancer has always represented a clinical challenge. Nearly all prostate biopsies are performed via the transrectal ultrasound (TRUS)-guided technique, which, however, suffers from multiple limitations owing to its inability to accurately visualize and target suspicious lesions. Advances in prostate MR imaging allow for direct visualization of suspicious regions of the prostate gland, opening the door for lesion-directed biopsy techniques. Advancing biopsy techniques include direct MR imaging guidance and MR imaging/ultrasound fusion.
Key points
- •
A diagnosis of localized prostate cancer has always represented a clinical challenge.
- •
Nearly all prostate biopsies are performed via the transrectal ultrasound-guided (TRUS) technique, which suffers from multiple limitations owing to its inability to accurately visualize and target suspicious lesions.
- •
Advances in prostate MR imaging now allow for direct visualization of suspicious regions of the prostate gland, opening the door for lesion-directed biopsy techniques.
- •
Advancing biopsy techniques include direct MR imaging guidance and MR imaging/ultrasound fusion.
Introduction
Prostate cancer is the second most frequently diagnosed cancer worldwide and the sixth leading cause of cancer death in men, accounting for 14% of total new cancer cases and 6% of total cancer deaths. Fortunately, it is now estimated that 92% of new cases of prostate cancer are clinically localized at diagnosis, for which the 5-year relative survival approaches 100%. Unfortunately, current state-of-the-art diagnostic and staging algorithms that are based on TRUS biopsies have substantial limitations resulting in unnecessary biopsies, inaccurate characterization of prostate cancer aggressiveness, patient anxiety, morbidity, and increased cost. Optimizing treatment strategies requires a careful establishment of an individual’s prognosis to avoid unnecessary therapy-induced morbidity or treatment failure. Fundamental to this effort is the ability to achieve a reasonable degree of accuracy for preoperative staging. Initially in this report, current methods and recommendations for prostate screening are discussed. A discussion regarding current paradigms in prostate cancer biopsy ensues.
The two most commonly used tests for diagnosis of prostate cancer are serum prostate-specific antigen (PSA) level measurement and digital rectal examination (DRE). However, current recommendations are evolving given the controversies surrounding the potential benefits and limitations of PSA testing. The American Urological Association (AUA) currently recommends an individualized approach in concert with shared decision making with regards to screening men between 40 and 69 years of age. Individuals who are at high risk (ie, positive family history or African American race) can begin screening at age 40 if both physician and patient agree on the risks and benefits. The same principles apply to patients between 55 and 69 years. The AUA does not currently recommend routine screening of individuals older than 70 years of age or any man with a less than 10- to 15-year life expectancy.
When prostate cancer is suspected either on the basis of elevated serum PSA levels or abnormal DRE, the diagnosis must be confirmed via biopsy. Prostate biopsies to diagnose or exclude cancer are currently performed approximately 1 million times annually in the United States. Nearly all are performed using the TRUS technique, initially introduced approximately 25 years ago. This technique is performed without knowing the exact tumor location within the prostate (blind biopsy). Currently, prostate cancer is the only major cancer in which diagnosis is routinely made with a blind biopsy of the organ. Unfortunately, microfocal cancers of little clinical significance are frequently detected with blind biopsies. Conversely, for first-time patients, the incidence of false-negative biopsies (ie, serious tumors not detected) may in first-time biopsies be as high as 35%. In addition, the prostate cancer detection rate of TRUS-guided biopsy decreases with every repeat biopsy and increases with the number of cores collected. The increase in detection rate could be attributed to better systematic sampling approach of TRUS biopsies. In comparison, cancer detection rates of targeted biopsy techniques, such as MR imaging or MR imaging/TRUS–directed biopsies, have been shown to be not affected by prior biopsies.
After a diagnosis of low-risk localized prostate cancer (based on clinical stage, PSA, and Gleason grade), approximately 90% of patients elect definitive treatment. This includes either surgery or radiation; the remaining 10% of men choose an active surveillance plan. Active surveillance refers to the close monitoring of patients with favorable-risk prostate cancer by keeping track of serial PSAs, having regular DREs, and undergoing periodic biopsies. Appropriate treatment is provided to active surveillance patients who show evidence of disease progression (either increase tumor volume or increase in Gleason grade). Studies show that prior to initiating patients into active surveillance, a restaging biopsy of the prostate improves selection by excluding up to 30% of those with higher volume or higher stage/grade disease. Thus, accurate disease characterization at diagnosis is paramount.
Multiparametric MR imaging of the prostate is capable of detecting clinically relevant prostate cancer. Although data currently suggest that MR imaging has the potential to assess the biologic aggressiveness of the tumor, it does not replace the need for biopsy histopathologic verification at this time. Instead, it is imperative to obtain biopsies of tumor-suspicious areas within the prostate using a combination of TRUS and MR imaging guidance. A recent systematic review showed that the efficiency of the targeted sampling technique is superior to the standard approach of blind biopsies (70% vs 40%).
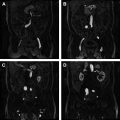
All MR imaging–guided prostate biopsies begin with a diagnostic prostate MR. According to the European Society of Urogenital Radiology “Prostate MR Guidelines 2012,” this includes a combination of a T2-weighted sequence and 2 functional sequences (diffusion-weighted images with either a dynamic contrast-enhanced MR imaging sequence or MR imaging spectroscopy) ( Fig. 1 ).
High-quality diagnostic preprocedural prostate MR imaging is essential for accurate biopsy planning.
Introduction
Prostate cancer is the second most frequently diagnosed cancer worldwide and the sixth leading cause of cancer death in men, accounting for 14% of total new cancer cases and 6% of total cancer deaths. Fortunately, it is now estimated that 92% of new cases of prostate cancer are clinically localized at diagnosis, for which the 5-year relative survival approaches 100%. Unfortunately, current state-of-the-art diagnostic and staging algorithms that are based on TRUS biopsies have substantial limitations resulting in unnecessary biopsies, inaccurate characterization of prostate cancer aggressiveness, patient anxiety, morbidity, and increased cost. Optimizing treatment strategies requires a careful establishment of an individual’s prognosis to avoid unnecessary therapy-induced morbidity or treatment failure. Fundamental to this effort is the ability to achieve a reasonable degree of accuracy for preoperative staging. Initially in this report, current methods and recommendations for prostate screening are discussed. A discussion regarding current paradigms in prostate cancer biopsy ensues.
The two most commonly used tests for diagnosis of prostate cancer are serum prostate-specific antigen (PSA) level measurement and digital rectal examination (DRE). However, current recommendations are evolving given the controversies surrounding the potential benefits and limitations of PSA testing. The American Urological Association (AUA) currently recommends an individualized approach in concert with shared decision making with regards to screening men between 40 and 69 years of age. Individuals who are at high risk (ie, positive family history or African American race) can begin screening at age 40 if both physician and patient agree on the risks and benefits. The same principles apply to patients between 55 and 69 years. The AUA does not currently recommend routine screening of individuals older than 70 years of age or any man with a less than 10- to 15-year life expectancy.
When prostate cancer is suspected either on the basis of elevated serum PSA levels or abnormal DRE, the diagnosis must be confirmed via biopsy. Prostate biopsies to diagnose or exclude cancer are currently performed approximately 1 million times annually in the United States. Nearly all are performed using the TRUS technique, initially introduced approximately 25 years ago. This technique is performed without knowing the exact tumor location within the prostate (blind biopsy). Currently, prostate cancer is the only major cancer in which diagnosis is routinely made with a blind biopsy of the organ. Unfortunately, microfocal cancers of little clinical significance are frequently detected with blind biopsies. Conversely, for first-time patients, the incidence of false-negative biopsies (ie, serious tumors not detected) may in first-time biopsies be as high as 35%. In addition, the prostate cancer detection rate of TRUS-guided biopsy decreases with every repeat biopsy and increases with the number of cores collected. The increase in detection rate could be attributed to better systematic sampling approach of TRUS biopsies. In comparison, cancer detection rates of targeted biopsy techniques, such as MR imaging or MR imaging/TRUS–directed biopsies, have been shown to be not affected by prior biopsies.
After a diagnosis of low-risk localized prostate cancer (based on clinical stage, PSA, and Gleason grade), approximately 90% of patients elect definitive treatment. This includes either surgery or radiation; the remaining 10% of men choose an active surveillance plan. Active surveillance refers to the close monitoring of patients with favorable-risk prostate cancer by keeping track of serial PSAs, having regular DREs, and undergoing periodic biopsies. Appropriate treatment is provided to active surveillance patients who show evidence of disease progression (either increase tumor volume or increase in Gleason grade). Studies show that prior to initiating patients into active surveillance, a restaging biopsy of the prostate improves selection by excluding up to 30% of those with higher volume or higher stage/grade disease. Thus, accurate disease characterization at diagnosis is paramount.
Multiparametric MR imaging of the prostate is capable of detecting clinically relevant prostate cancer. Although data currently suggest that MR imaging has the potential to assess the biologic aggressiveness of the tumor, it does not replace the need for biopsy histopathologic verification at this time. Instead, it is imperative to obtain biopsies of tumor-suspicious areas within the prostate using a combination of TRUS and MR imaging guidance. A recent systematic review showed that the efficiency of the targeted sampling technique is superior to the standard approach of blind biopsies (70% vs 40%).
All MR imaging–guided prostate biopsies begin with a diagnostic prostate MR. According to the European Society of Urogenital Radiology “Prostate MR Guidelines 2012,” this includes a combination of a T2-weighted sequence and 2 functional sequences (diffusion-weighted images with either a dynamic contrast-enhanced MR imaging sequence or MR imaging spectroscopy) ( Fig. 1 ).
High-quality diagnostic preprocedural prostate MR imaging is essential for accurate biopsy planning.
Methods of MR imaging–guided prostate biopsy
Three methods of MR imaging guidance are currently available for performance of targeted prostate biopsies cognitive fusion, direct MR imaging–guided biopsy, and software coregistration of a stored MR imaging with real-time ultrasound using a fusion device. Each method has its advantages and disadvantages.
Cognitive Fusion
Cognitive fusion is simple, quick, and requires no additional equipment beyond MR imaging and a conventional TRUS facility. Suspicious areas detected on a prior diagnostic MR imaging are biopsied using TRUS. Because suspicious lesions are localized and detected on MR imaging, targeted biopsy of the corresponding region on TRUS is expected to yield better outcomes than random, blind biopsies of the prostate. Specialized training beyond conventional TRUS biopsy is not required for an ultrasound operator. One recent study suggests that cognitive fusion yields improved accuracy over conventional systematic blind biopsy. A disadvantage of cognitive fusion is the potential for human error in the extrapolation from MR imaging to TRUS without an actual overlay for targeting.
MR Imaging–Guided Prostate Biopsy
The ability to visualize suspicious prostate areas on MR imaging allows for lesion-directed biopsy under MR imaging guidance. This is possible because of the drastically increased speed of MR imaging in the past two decades along with recent advances in computer-based accurate biopsy needle guidance tools. This provides the individual performing the procedure the ability to track the path of the biopsy needle and confirm its position in the lesion of interest prior to performing the biopsy.
MR imaging–guided prostate biopsies have been performed in low-field open MR imaging and the widely available closed-bore MR imaging scanners with 1.5-T or 3-T field strengths. The low-field MR imaging allows for easier access to patients, whereas the closed-bore scanner offers a much higher signal-to-noise ratio, allowing for better visualization of the lesion. As for the biopsy approach, a transrectal approach is the preferred method given that it is considered less invasive.
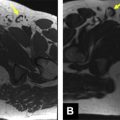
The MR imaging–guided biopsy is generally performed on a separate date from the diagnostic planning MR imaging. It can follow a same-day diagnostic study if desired. Oral fluoroquinolones are given before and after the biopsy. The actual MR imaging–guided biopsy is performed in bore, or within the MR imaging tube. Patients are generally placed in the prone position. A body phased-array or cardiac coil is placed on the patient’s lower back and the MR biopsy device is inserted into the rectum via the endorectal needle guide ( Fig. 2 ). Multiplanar localization sequences are performed to identify the regions of interest, typically using a T2-weighted fast spin-echo sequence. The gadolinium-filled needle guide is then directed toward the lesion by means of the prostate biopsy device and guidance sequences are performed between needle guide adjustments. Automated software assists needle placement by providing automated adjustment parameters for the needle ( Fig. 3 ). The guidance sequences used are usually T2-weighted fast spin-echo or single-shot fast spin-echo obtained either in the sagittal or oblique axial planes that contain the needle.