Because of its high negative predictive value in excluding breast cancer, magnetic resonance (MR) imaging plays a role in the evaluation of selected clinical and imaging findings of the breast, especially when biopsy is not technically feasible. Case selection is very important in ensuring the efficacy of this use of MR imaging because of potential false-positive and (albeit less likely) false-negative results. This article examines the clinical scenarios and imaging findings in which MR imaging is contributory to patient management after conventional workup with equivocal results.
Mammography, sonography, and magnetic resonance (MR) imaging are established imaging modalities for detecting breast cancer. While MR imaging is known to be a sensitive method for depicting breast cancer, its precise range of clinical applications remains controversial, especially in the era of cost containment. The costs of MR imaging include not only the scan itself but also the downstream costs of evaluating incidental lesions and false-positive results, leading to financial costs, patient anxiety, and potential morbidity from biopsy and intravenous gadolinium administration. A meta-analysis of 44 studies supported the commonly accepted notion that breast MR imaging is associated with a high sensitivity but relatively limited specificity. Nevertheless, MR imaging tends to have a higher negative predictive value than conventional breast imaging studies, such as mammography and ultrasound.
It is important to define the appropriate applications of MR imaging in breast imaging to optimize its clinical use. In 2008, the American College of Radiology published guidelines for clinical applications of breast MR imaging. These guidelines include:
- 1.
Screening of high-risk patients, the contralateral breast in patients with recent diagnosis of breast cancer, and patients with augmented or reconstructed breasts.
- 2.
Defining extent of disease after diagnosis of invasive carcinoma and/or ductal carcinoma in situ (DCIS), including relationship of disease to the fascia, pectoralis major, serratus anterior, and/or intercostals muscles, residual disease in patients after lumpectomy with positive margins, and monitoring of neoadjuvant chemotherapy patients.
- 3.
Additional evaluation of clinical or imaging findings, including tumor recurrence at lumpectomy site, metastatic axillary lymphadenopathy of unknown primary, lesion characterization when mammography, ultrasound, and physical examination findings are equivocal and biopsy is not possible, and suspected tumor in reconstructed breast.
A similar consensus document was published in Europe in 2007. This group specifically contended that MR imaging should not be used as a diagnostic tool in the setting of equivocal findings at conventional imaging if biopsy (especially percutaneous core biopsy) could be performed for tissue diagnosis.
This review focuses on the use of MR imaging in the additional evaluation of clinical or imaging findings. In the setting of a worrisome or suspicious clinical or imaging finding where biopsy is possible, it is well accepted that MR imaging cannot negate the need for biopsy. However, MR imaging may be useful in solving remaining questions after thorough assessment using mammography and sonography, especially when biopsy is not technically possible. Such use falls into 2 categories:
- 1.
Clinical problems that cannot be sufficiently addressed with conventional imaging methods such as mammography and ultrasound.
- 2.
Imaging findings that are indeterminate despite thorough evaluation.
A high negative predictive value is the underlying premise for this use of MR imaging. In other words, even when MR imaging does not provide a specific answer, it may be used to exclude malignancy in many circumstances. To realize the high negative predictive value of MR imaging, radiologists must optimize all MR imaging parameters, including imaging equipment and technique, interpretation criteria, and correlation with mammography and ultrasound.
Clinical symptoms
At this time, MR imaging has a limited role in the evaluation of clinical symptoms in the absence of suspicious imaging findings, with the exception of assessing patients who present with nipple discharge.
Palpable Lump
A palpable lump is a very common clinical complaint. Although most palpable lumps do not represent breast cancer, it is the most common clinical symptom of breast cancer. Therefore, it is important to properly evaluate palpable lumps, both to diagnose breast cancer in a timely fashion and to accomplish this goal in a cost-effective manner. In the setting of a clinically significant palpable lump, diagnostic mammography and ultrasound should be performed as the initial examinations. The combination of properly performed diagnostic mammography and ultrasound carries a very high negative predictive value (nearly 100%). In the setting of a suspicious imaging finding or persistent clinical suspicion (despite lack of an imaging correlate), tissue diagnosis via percutaneous core biopsy is often performed. Although MR imaging may depict the palpable lump in question, diagnostic mammography and ultrasound are more efficacious and cost-effective methods for evaluation. The American College of Radiology (ACR) Appropriateness Criteria rated MR imaging as very low in appropriateness in the initial evaluation of palpable breast masses. On a scale of 1 to 9 (1 = least appropriate; 9 = most appropriate), MR imaging was given a rating of 1, whereas diagnostic mammography and ultrasound were each given a rating of 9.
Breast Pain
Although pain (both focal and diffuse) is the most common breast complaint, there are no data to support the use of MR imaging for evaluation of this symptom, especially in the absence of a palpable lump or other clinical findings of breast cancer. Unilateral, focal, noncyclical pain is of greater clinical concern than bilateral, diffuse, cyclical pain that waxes and wanes with the menstrual cycle, which is almost always benign. A study of 110 targeted ultrasound examinations in 99 patients who presented with focal breast pain (in the absence of an associated palpable finding) did not reveal any cancers. It was concluded that ultrasound (and perhaps other imaging studies) is most useful for patient reassurance in this setting, rather than for diagnosis of breast cancer. Similarly, MR imaging has no particular role in evaluating patients with breast pain, particularly given its high costs and relatively limited specificity.
Nipple Discharge
Of all clinical symptoms in the breast, pathologic nipple discharge is the symptom with the best documented diagnostic role for MR imaging. Investigators have advocated this use of MR imaging because of its high diagnostic performance, even when conventional imaging modalities are negative. At the Consensus Conference of the Congress “Attualita in Senologia” in 2007, the Italian scientific societies supported this use of MR imaging when mammography, ultrasound, and galactography results are negative or ambiguous.
Nipple discharge is most significant when it is bloody (serosanguinous) or spontaneous. In the majority of cases, even if bloody and spontaneous, nipple discharge stems from a benign cause. Most (80%–90%) cases of nipple discharge result from ductal ectasia and/or papillomas. Although invasive, surgical duct exploration remains the definitive method of evaluation. Imaging studies are primarily performed to establish or exclude the presence of lesions (both benign and malignant). Exact diagnosis at imaging is often not possible because many lesions have overlapping, nonspecific imaging findings.
The optimal imaging workup protocol for nipple discharge remains controversial. Galactography has traditionally been performed. However, this requires duct cannulation and may be both technically challenging, with up to 10% failure rates even when performed by experienced practitioners, and uncomfortable for the patient. Furthermore, the diagnostic information that this study yields may be limiting. The reported sensitivities of galactography for cancer ranged between 0% and 55%. Although preoperative wire localization is possible, it remains technically challenging. In up to 20% of cases where galactography revealed a lesion, subsequent surgical excision was unsuccessful.
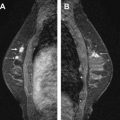
In a study of 23 patients with nipple discharge who had undergone MR imaging evaluation, Orel and colleagues found that 73% of the MR imaging findings correlated with pathology and concluded that MR imaging may serve as a noninvasive method of duct evaluation (instead of galactography). Similarly, Ballesio and colleagues examined 44 patients and reported that MR imaging was able to show the galactographic findings. Nakahara and colleagues compared ultrasound, galactography, and MR imaging in 55 patients and found that MR imaging showed the location and distribution of lesions best, especially in cases of DCIS. Although galactography is sensitive in depicting papillomas, MR imaging was found to be better in demonstrating the location and distribution of lesions, particularly important in processes (such as DCIS or papillomatosis) that may extend over a large region ( Fig. 1 ).
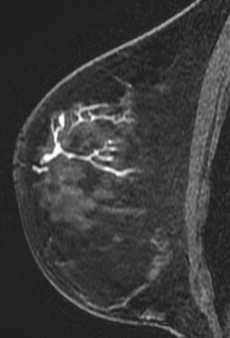
The use of MR imaging in evaluating patients with nipple discharge and negative conventional imaging has also been investigated. Specificity remains limited, and differentiation between benign papilloma and malignancy (either invasive or in situ) is usually not possible.
MR imaging galactography is a novel imaging test consisting of indirect and direct MR galactography. In the former setting, heavily T2-weighted sequences are used, with no contrast agent. Similar to conventional galactography, dilated ducts are seen as tubular structures with high signal intensity. Lesions may be seen as defects in intraductal signal, duct wall irregularity, or ductal obstruction.
Direct MR galactography (also known as MR contrast galactography) requires cannulation of the ducts and injection of contrast agent. In a pilot study of 16 patients, gadopentate dimeglumine (0.1 mL) was mixed with nonionic contrast material (0.9 mL), and the resultant 1-mL solution was injected using a 30-gauge needle, on cannulation of the secreting ducts. Three-dimensional gradient-echo fat-saturated turboflash sequences were used for imaging. These same patients also underwent conventional galactography. When compared with surgical and histopathologic results, MR contrast galactography was found to be just as sensitive, but with fewer false positives than conventional galactography.
Schwab and colleagues compared direct MR galactography (using gadopentetate dimeglumine), indirect MR galactography, and conventional galactography in 23 patients with pathologic nipple discharge. At conventional galactography, 57 lesions were identified. Direct MR galactography was more sensitive than indirect galactography when using conventional galactography results as the gold standard.
Similar to conventional galactography, visualization of the extent of disease is limited with MR galactography (both indirect and direct). Therefore, fusion of MR ductography and MR images of the breasts has been proposed to identify the relationship between the intraductal abnormality and extent of disease.
Equivocal imaging findings
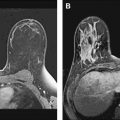
MR imaging has long been used for additional assessment of indeterminate imaging findings. Nevertheless, this clinical application has been relatively limited, as percutaneous imaging-guided core biopsy is both safe and efficacious, and provides a definitive histologic diagnosis in most cases. However, tissue diagnosis is sometimes not indicated or technically possible. One of the biggest challenges in mammography is the distinction between superimposition of fibroglandular structures (summation artifact) from a true lesion ( Fig. 2 ). In other words, one must first determine whether a potential lesion is even real before proceeding farther in the workup. This determination is a prerequisite in biopsy evaluation because biopsy of summation artifact is not indicated. Even when a true lesion is suspected, ideally it should be identified with certainty in 2 projections, preferably orthogonal, before performance of ultrasound or biopsy. Absence of a sonographic correlate or lesion identification in 2 mammographic projections diminishes the likelihood of success of core biopsy and sometimes renders core biopsy technically impossible. Surgical excision is also limited when preoperative wire localization cannot be performed with confidence in 2 mammographic projections.
The question arises as to whether or not MR imaging has a sufficiently high negative predictive value to exclude malignancy when biopsy is not possible. Given the high sensitivity of this modality, high negative predictive values of approximately 98% have been reported. These numbers are higher than those reported for other adjunctive breast imaging tests, including nuclear medicine studies of the breasts (positron emission tomography, positron emission mammography, and scintimammography). It remains to be demonstrated whether these high negative predictive values reported in single-institution studies from academic centers can be replicated and generalized in the community. An overall negative predictive value of 85.4% was reported in a multi-institutional trial. This number is lower than the traditionally reported negative predictive value of breast MR imaging (usually more than 90%) and likely too low for MR imaging to be used to obviate biopsy. In addition to optimal technique and radiologist interpretation, it appears that case selection is a very important variable in ensuring that MR imaging is of clinical benefit in evaluating equivocal imaging findings.
MR imaging should only be used as a problem-solving tool after full and detailed mammographic assessment. In one of the largest studies to date on the use of MR imaging in evaluating equivocal mammographic findings, Moy and colleagues was careful in the study design to include only cases that had been thoroughly evaluated with supplemental mammographic views (especially when attempting to localize potential lesions in 2 mammographic projections), including true lateral, spot-compression, magnification, rolled or exaggerated craniocaudal, step-oblique views, or a combination of these views.
Most lesions and most cancers can be identified in 2 mammographic projections. However, there is a small subset of cancers that are seen only or primarily in one view. The challenge in evaluating one-view-only imaging findings is that malignancy is highly unlikely, but possible. Sickles reviewed 61,273 screening mammography examinations and identified 2023 (3.3%) studies in which findings were prospectively identified in only one standard mammographic projection (mediolateral oblique or craniocaudal). The majority (82.7%) of the cases was considered to represent summation artifact, with this assessment made at either the time of initial screening interpretation (53.7%) or subsequently after recall imaging (29.0%). Of the remaining cases, 36 cancers were identified. Invasive lobular carcinoma was disproportionately represented in this series, constituting 33% of cancers presenting in one view versus 10% of all cancers from the same institution. This trend has also been noted by other investigators.
Diagnostic mammography results in identification of the majority of true lesions (and cancers) in 2 projections. However, there remains a small subset of true lesions (and cancers) that cannot be well visualized in 2 projections despite tailored diagnostic views. Before application of MR imaging in such cases, ultrasound should be part of the initial imaging evaluation. Ultrasound is a very helpful tool in assessing mammographic lesions, particularly in patients with dense breasts. However, ultrasound can be limited if the lesion in question has not been identified with certainty in 2 projections. In the study by Moy and colleagues, ultrasound was performed as part of the initial imaging evaluation in 72.8% of the study cohort with dense breasts (heterogeneously dense or extremely dense) and 17.6% of those with less dense breasts (scattered fibroglandular densities). In the series of 86 lesions by Lee and colleagues, ultrasound was performed in 47 (55%) of cases. Ultrasound was not performed in the remaining 45% of cases because of the vagueness of the mammographic finding, lack of accuracy in localization, and inability of ultrasound to distinguish between scar and tumor.
Lesion Type
Cancer may be depicted at mammography as mass, calcifications, architectural distortion, or asymmetry. Because case selection is of utmost importance in ensuring the efficacious use of MR imaging in the evaluation of equivocal imaging findings, MR imaging should only be performed in this context for specific lesion types. In general, it is not indicated as a problem-solving modality after identification of mass or calcifications.
Mass
A mass differs from an asymmetry because a mass is a finding that is seen in 2 (or more) projections, characterized by convex outward margins and associated with central density. In many cases, there is a sonographic correlate to the mammographic mass. Biopsy may then be performed using core biopsy (using stereotaxis or ultrasound guidance) or surgical excision (preoperative wire localization may be performed using mammographic or ultrasound guidance) as indicated. Although MR imaging may reveal a correlate to the mammographic mass, it is not indicated because it cannot be used in lieu of a biopsy in most cases. Indeed, the ACR Appropriateness Criteria rated MR imaging as very low in appropriateness in the evaluation of nonpalpable breast masses.
Calcifications
Calcifications may form in breast cancers as a result of dystrophic reaction associated with cell turnover. At the same time, many benign forms of calcifications occur in the breasts. Although linear branching pleomorphic calcifications are characteristic of malignancy, many calcifications are indeterminate, with positive predictive value of malignancy at biopsy of only 21.7% reported in one series. MR imaging has been used in some clinical situations to preclude the need for biopsy, particularly in cases of low-suspicion calcifications, such as amorphous calcifications. However, even in cases of amorphous calcifications the probability of malignancy is 20%.
MR imaging is limited in the additional evaluation of calcifications beyond mammography (using fine-detailed magnification views) because magnetic resonance does not depict calcifications as well as mammography. Furthermore, malignant calcifications are mostly associated with the diagnosis of DCIS rather than invasive cancer, and the sensitivity of MR imaging for DCIS is lower than its sensitivity for invasive cancer, particularly in cases of low-grade DCIS. Therefore, the negative predictive value of MR imaging in the setting of suspicious calcifications at mammography is only approximately 85%, making it insufficient to exclude malignancy. In a multi-institutional Italian trial consisting of 112 cases of calcifications for which biopsy was recommended (assessed as Breast Imaging Reporting and Data System BI-RADS 4, suspicious or BI-RADS 5, highly suggestive of malignancy), the negative predictive value of MR imaging was only 71%. Similarly, in a single-institution study of 55 women with BI-RADS 3 (probably benign), BI-RADS 4 (suspicious), or BI-RADS 5 (highly suggestive of malignancy) calcifications, the reported negative predictive value was 76%.
Therefore, MR imaging cannot be performed as an alternative to biopsy in the setting of suspicious calcifications at mammography. The ACR Appropriateness Criteria rated MR imaging as very low in appropriateness in the evaluation of breast calcifications. After a diagnosis of in-situ cancer is made in the setting of mammographically detected calcifications, MR imaging may play a role in identifying any invasive component and to delineate the extent of disease, especially if DCIS is high grade.
Architectural distortion
Architectural distortion represents distortion of tissue and may be seen as white lines emanating from a center without a central mass. The most common cause is surgical scarring, but it may result from the desmoplastic reaction that some cancers exert on adjacent tissues. In the absence of a sonographic correlate, management of the finding of architectural distortion may be challenging. Stereotactic-guided core biopsy would be technically challenging, as architectural distortion lacks a central density and may not be identifiable without sufficient compression. Wire-localization surgical excision, besides being invasive, would require confident identification of the finding in 2 (preferably orthogonal) mammographic views.
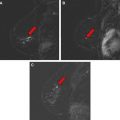
Benign causes for architectural distortion at mammography include radial scar (complex sclerosing lesion) and sclerosing adenosis. However, in the absence of clinical history that suggests scarring (such as history of surgery), architectural distortion is considered a suspicious finding that requires biopsy ( Fig. 3 ). The challenge occurs when it is only identified in one view and when there is no sonographic correlate; biopsy (either core or surgical excision) would not be technically feasible. MR imaging may have a role in such cases, but there are insufficient data currently to support or negate this potential role.
Perfetto and colleagues examined the MR imaging results in 20 patients with focal architectural distortion at mammography. Their study investigated whether or not MR imaging can be used to determine which patients need to proceed to surgical excision (vs imaging follow-up). Twenty-four mammographic lesions were present in these 20 patients. It is unknown whether these mammographic lesions were identified in 1 or 2 views. At mammography, 8 of these 24 mammographic lesions were assessed as BI-RADS 3 (probably benign), 8 as BI-RADS 4 (suspicious), and 8 as BI-RADS 5 (highly suggestive of malignancy). At MR imaging, 24 lesions were identified, 7 of which were cancers. The investigators reported a high negative predictive value (100%) in that no cancers were missed without MR imaging showing malignant features. Although this was a small study, one may infer from its results that MR imaging may play a role in excluding cancer in cases of architectural distortion where biopsy is not technically feasible.
Asymmetry
Mammographic asymmetry differs from a mass in that it is characterized by concave (rather than convex) outward margins and may be interspersed with fat. According to the most current edition of Breast Imaging Reporting and Data System, there are 3 types of asymmetries :
- 1.
Asymmetry (see Fig. 2 )
- 2.
Global asymmetry
- 3.
Focal asymmetry.
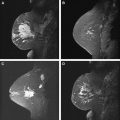
An asymmetry is a finding seen primarily in one view, whereas a focal asymmetry is seen in 2 views. Global asymmetry is diffuse increase in breast tissue in one breast when compared with the other breast. In addition to these 3 types of asymmetries, the term developing asymmetry has been defined as a focal asymmetry that is new or increasing in size or conspicuity when compared with a prior mammographic examination ( Figs. 4 and 5 ). Of all mammographic lesions, MR imaging has demonstrated the greatest clinical utility in assessing asymmetries (vs mass, calcifications, or architectural distortion).
The probability of malignancy ranges from 12.8% for developing asymmetry to 1.8% for one-view asymmetry to 0% for nonpalpable global asymmetry. The role of MR imaging has only been studied peripherally in studies of asymmetries because these lesions occur infrequently, so an exact role for MR imaging has yet to be established. In a study of 311 cases of developing asymmetries obtained from a cohort of 180,801 consecutive screening and 27,330 consecutive diagnostic mammography examinations, MR imaging was performed in only 2 cases. In one case, MR imaging showed nonmass enhancement with plateau kinetics as the correlate to the developing asymmetry, and biopsy revealed pseudoangiomatous stromal hyperplasia. In the second case, MR imaging was negative (with no correlate), and the patient was cancer-free at follow-up.
Empirical Data
To date, there are only a small number of well-controlled studies on the use of MR imaging in resolving equivocal imaging findings, partly because biopsy, rather than MR imaging, is performed in many circumstances. Comparison of the major studies is limited given that each study was designed in a slightly different way and focused on different parameters. Given that the equivocal imaging findings that may benefit from MR imaging occur only infrequently, multi-institutional trials may be necessary with large enough sample sizes to produce results that can be generalized to different centers.
In the 1990s, Orel and colleagues reported the use of MR imaging in assessing one-view mammographic findings or equivocal changes in sites of prior biopsy. Sardanelli and colleagues studied 19 cancers with inconclusive mammographic findings that had been imaged with MR imaging, reported 1 false-negative and 2 false-positive MR imaging examinations, and concluded that MR imaging was helpful.
Lee and colleagues retrospectively identified 86 lesions that were evaluated by MR imaging because of equivocal findings at mammography. Inclusion criteria for this use included:
- 1.
Definite mammographic finding identified but its significance was unclear (n = 26)
- 2.
Inconclusive mammographic finding (with or without addition of ultrasound) as to whether a true lesion existed (n = 20)
- 3.
Suspicious finding identified but its true location was uncertain (n = 16)
- 4.
Possible mammographic change representing tumor recurrence versus posttreatment change in patients who had undergone breast conservation therapy (n = 15)
- 5.
Increased density or distortion at site of prior benign biopsy (n = 9).
Of these 86 lesions, 58 (67%) were breast asymmetries, and 28 (33%) were cases of architectural distortion.
Contrast-enhanced MR imaging using 1.5-Tesla magnet was performed. The earlier examinations during the study period were performed using a 2-dimensional spin echo sequence; the later examinations were performed using a 3-dimensional fast spoiled gradient sequence with fat suppression. MR imaging was considered positive if focal enhancement (either mass or nonmass) was identified. MR imaging was considered negative if there was no enhancement, or if enhancement was either diffuse and uniform or similar to that of background parenchyma. The MR imaging finding was considered a correlate if it accounted for the mammographic finding based on location; otherwise, it was considered an incidental finding. Twenty-six (30%) correlates were identified at MR imaging, along with 12 incidental lesions. All 26 correlates were biopsied, and 9 cancers were diagnosed. No cancer was identified in the remaining 60 mammographic lesions that had no correlate at MR imaging, 6 of which were excised with benign results, and 54 of which were stable at mammographic follow-up (mean, 19 months).
The largest series on the use of MR imaging in evaluating equivocal imaging findings was published in 2009. Moy and colleagues retrospectively reviewed 115 MR imaging examinations that were performed after inconclusive mammographic findings: asymmetries without associated microcalcifications (n = 98, 85.2%), architectural distortion (n = 12, 10.4%), and change at prior benign biopsy site (n = 5, 4.3%). MR imaging was performed using T1-weighted fat-suppressed 3-dimensional fast spoiled recalled echo sequence. No suspicious MR imaging correlate was present in 100 (87%) of 115 cases. These negative cases were stable at imaging (either mammography or MR imaging) follow-up, with mean follow-up interval of 34 months. In 15 (13%) of the 115 cases, enhancing masses were identified that corresponded to the mammographic finding. At biopsy, 6 of the 15 masses were malignant. Of these 6 cancers, 4 were seen in one mammographic view only. Although none were identified at initial ultrasound, 2 of the 6 cancers were identified at second-look ultrasound. When compared with mammography, MR imaging had a higher specificity (91.7% vs 80.7%, P = .029), positive predictive value (40% vs 8.7%, P = .032), and accuracy (92.2% vs 78.3%, P = .0052). Because MR imaging identified all 6 cancers, its sensitivity in this series was 100%.
In contrast to the study by Lee and colleagues, Moy and colleagues excluded cases of mammographic changes at the lumpectomy site because the utility of MR imaging for differentiating tumor recurrence versus surgical scar had already been proven by other published studies. Also excluded were suspicious lesions amenable to mammographic or sonographic localization for biopsy, suspicious calcifications, and questionable findings correlating with palpable lumps. In other words, the study by Moy and colleagues sought to address the utility of MR imaging in evaluating equivocal imaging questions in a cohort in which biopsy was not possible.
Surgical Scar
Surgical scars are often associated with spiculation and tissue distortion at mammography, making it difficult to distinguish from tumor, either recurrent after lumpectomy, or new in a site of prior benign surgical biopsy. Ultrasound is often challenging as well, as scars are often associated with significant posterior acoustic shadowing and variable echogenicity and may mimic cancers. Clinical examination is also of limited utility because scarring and tumor may not be discernible when the clinical presentation is induration and palpable thickening.
On the other hand, MR imaging is particularly useful for distinguishing scar tissue from recurrent tumor ( Fig. 6 ). This is one area, among the various equivocal imaging findings, where MR imaging has been proven to be of significant clinical use. If performed and interpreted properly, MR imaging is both sensitive and specific in distinguishing recurrent tumor from surgical scar. Optimal technique is required for this distinction, as kinetic evaluation plays a significant role. In a study of 20 women with suspected tumor recurrence after breast conservation surgery, Kerslake and colleagues found that benign scars enhance slowly and less strongly, whereas tumor recurrence demonstrated rapid, early, and avid enhancement.