MRI has become the most important imaging modality for detecting and characterizing focal liver lesions. The introduction of high-field-strengths, such as 3 Tesla MR imaging, in combination with the parallel imaging technique, has led to significant improvements in spatial and temporal resolution and has established this technique as a valuable asset in daily clinical practice. New techniques, such as diffusion-weighted imaging, may improve MR imaging sensitivity and specificity in the diagnostic workup of focal liver lesions. The tailored administration of various nonspecific and liver-specific contrast agents enables clinicians to increase the detection rate and improve the characterization of the different focal liver lesions. This article describes the usefulness of these imaging techniques in detecting and characterizing the most common benign focal liver lesions.
Imaging of focal liver lesions
With the increased use of cross-sectional imaging modalities, focal liver lesions are more often detected incidentally or seen on surveillance scans, especially in patients with underlying oncologic diseases. Recent advances in CT and MR imaging technology allow the acquisition of thinner sections, resulting in the detection of hepatic lesions measuring in the millimeter range. Although a high prevalence of benign liver lesions measure 1 cm or less, substantial limitations remain regarding the confident characterization of these small hepatic lesions, particularly in patients with a clinical history of cancer. Furthermore, benign focal liver lesions can have an atypical appearance that can mimic metastases. Therefore, further characterization is desirable, because the presence of hepatic metastases or other malignant lesions may substantially alter patient prognosis and therapy. Given that most small focal liver lesions are benign in patients without obvious liver metastases, immediate further evaluation and definitive characterization of a benign lesion offer marked benefits beyond peace of mind for both patient and physician. Thus, from a clinical and socioeconomic point of view (eg, avoiding biopsy, multistep diagnosis, long-term follow-up), noninvasively establishing a confident diagnosis of focal liver lesions is very important.
Recent technical advances in the hard- and software of MR imaging technology, including the increased use of 3 Tesla MR imaging in the daily clinical routine, has led to better temporal and spatial resolution, particularly for contrast-enhanced, T1-weighted three-dimensional images, allowing near-isotropic imaging. Because the noninvasive characterization of focal liver lesions is largely based on their morphologic appearance or enhancement patterns on contrast-enhanced dynamic imaging, the use of various nonspecific and liver-specific contrast agents has significantly expanded the role of MR imaging in the diagnosis of focal liver lesions. These lesions can now be characterized based on their morphology, vascularity, and specific functional features on a cellular basis, through a tailored examination that leads to a confident and noninvasive diagnosis. The introduction of new MR imaging pulse sequences, such as diffusion-weighted imaging (DWI), into routine clinical practice may further improve the noninvasive diagnostic workup of focal liver lesions.
This article describes the most commonly encountered benign focal liver lesions, including simple cysts, biliary hamartoma, hemangioma, focal nodular hyperplasia (FNH), and adenoma. Their typical and atypical appearance and their enhancement pattern are illustrated, and how a confident noninvasive diagnosis can be achieved using the above-mentioned MR imaging techniques in combination with various available contrast agents is explained.
Technical considerations and contrast agents
Currently, liver MR imaging examinations are performed routinely using either 1.5 or 3 Tesla machines. Further recent developments in MR imaging technology include stronger gradient systems, multichannel coils, navigator triggering, and parallel imaging. Because of these remarkable technical innovations that lead to faster pulse sequences, the entire liver can be examined rapidly within a single breath-hold. Thus, multiphase, T1-weighted volume interpolated or three-dimensional gradient-echo (GRE), with almost isotropic voxel imaging and a slice thickness of 1 to 2 mm using VIBE (volumetric interpolated breath-hold examination) or THRIVE (T1W high-resolution isotropic volume examination), sequences for dynamic imaging of the liver and injection of gadolinium chelate contrast agent can be performed easily. This technique improves the detection and characterization of focal liver lesions based on their vascularity or typical enhancement pattern.
However, a high spatial resolution is indispensable for abdominal imaging, which necessitates longer acquisition times. New techniques to compensate for breathing motion artifacts, such as breath-triggering and navigator-triggering technology, offer a possible solution to this dilemma. Thus, high-resolution T2- and diffusion-weighted respiratory-triggered sequences are now feasible with increased high spatial and temporal resolution, leading to a marked improvement in the detection and characterization of focal liver lesions. The introduction of different liver-specific contrast agents has further established the role of MR imaging in liver imaging. Although dynamic gadolinium chelate–enhanced MR imaging is crucial in the detection and characterization of focal liver lesions, some limitations exist to the use of these contrast agents that have pharmacokinetics similar to those of the iodine contrast media used in CT.
Particular difficulties occur with liver tumors that show an atypical morphology and vascularity, or that show an overlapping enhancement pattern. Compared with extracellular contrast media, liver-specific MR contrast media are selectively taken up by the normal liver parenchyma through specific and well-known uptake mechanisms. Currently, two main groups of liver-specific contrast media are used in clinical practice. The first group includes superparamagnetic iron-oxide particles (SPIO) (ie, ferumoxide [Endorem], and ferucarbotran [Resovist]). These substances are transported out of the blood circulation and taken up by the reticuloendothelial system (or Kupffer cells of the liver) through phagocytosis. These particles cause magnetic field inhomogeneities with shortening of T2 relaxation time, leading to a decrease in the signal of the normal liver parenchyma on T2-weighted sequences, best seen on T2 or GRE sequences for lesion detection. However, for lesion characterization T2-weighted turbo spin-echo (TSE) sequences or HASTE (half-Fourier acquisition single-shot turbo spin-echo) sequences are more sensitive for evaluating signal intensity loss. Depending on the content of the Kupffer cells, benign hepatocellular lesions show uptake of these contrast agents. Hemangiomas present an exception for SPIO contrast media uptake because of their relatively large, slow-flowing blood pool that allows the effect of the SPIO particles to occur and therefore, although lacking Kupffer cells, show a decrease in signal on T2-weighted images.
Lesions that do not contain Kupffer cells (eg, malignant lesions, metastases) do not take up SPIO contrast agents, rendering them bright against the background of the dark liver parenchyma on T2-weighted post-SPIO sequences. The resulting increase in contrast between the liver and a lesion of nonhepatic origin increases the detection rate compared with unenhanced images and allows an easy distinction from benign hepatic lesions. Unfortunately, one of these agents (ferucarbotran) will no longer be available after the beginning of 2011.
The second group of liver-specific contrast agents includes the hepatobiliary contrast media, which are selectively taken up by hepatocytes and excreted through the biliary tracts. This activity results in an increase in signal of the normal liver parenchyma on T1-weighted sequences. Malignant lesions show washout, appearing hypointense, and thus become more conspicuous. Mangafodipir (Mn-DPDP), gadobenate dimeglumine (Gd-BOPTA), and gadoxetate (Gd-EOB-DTPA) belong to this group of contrast media. The manganese-based contrast agent Mn-DPDP (formerly known as mangafodipir trisodium or teslascan ) is a contrast medium that enhances the liver parenchyma and bile ducts through this pathway of excretion on T1-weighted sequences. This contrast agent was unfortunately discontinued recently. The gadolinium-based bimodal contrast agents show combined perfusion- and hepatocyte-specific properties. The subgroup of gadolinium-based contrast material comprises gadobenate dimeglumine (MultiHance, Bracco Diagnostics, Princeton, NJ, USA) and gadoxetate (Primovist, Bayer Schering Pharma, Berlin, Germany). These substances are administered intravenously as a bolus for dynamic imaging in the arterial, portal venous, and delayed phases, allowing the evaluation of morphology, vascularity, and functional properties within a single examination.
Technical considerations and contrast agents
Currently, liver MR imaging examinations are performed routinely using either 1.5 or 3 Tesla machines. Further recent developments in MR imaging technology include stronger gradient systems, multichannel coils, navigator triggering, and parallel imaging. Because of these remarkable technical innovations that lead to faster pulse sequences, the entire liver can be examined rapidly within a single breath-hold. Thus, multiphase, T1-weighted volume interpolated or three-dimensional gradient-echo (GRE), with almost isotropic voxel imaging and a slice thickness of 1 to 2 mm using VIBE (volumetric interpolated breath-hold examination) or THRIVE (T1W high-resolution isotropic volume examination), sequences for dynamic imaging of the liver and injection of gadolinium chelate contrast agent can be performed easily. This technique improves the detection and characterization of focal liver lesions based on their vascularity or typical enhancement pattern.
However, a high spatial resolution is indispensable for abdominal imaging, which necessitates longer acquisition times. New techniques to compensate for breathing motion artifacts, such as breath-triggering and navigator-triggering technology, offer a possible solution to this dilemma. Thus, high-resolution T2- and diffusion-weighted respiratory-triggered sequences are now feasible with increased high spatial and temporal resolution, leading to a marked improvement in the detection and characterization of focal liver lesions. The introduction of different liver-specific contrast agents has further established the role of MR imaging in liver imaging. Although dynamic gadolinium chelate–enhanced MR imaging is crucial in the detection and characterization of focal liver lesions, some limitations exist to the use of these contrast agents that have pharmacokinetics similar to those of the iodine contrast media used in CT.
Particular difficulties occur with liver tumors that show an atypical morphology and vascularity, or that show an overlapping enhancement pattern. Compared with extracellular contrast media, liver-specific MR contrast media are selectively taken up by the normal liver parenchyma through specific and well-known uptake mechanisms. Currently, two main groups of liver-specific contrast media are used in clinical practice. The first group includes superparamagnetic iron-oxide particles (SPIO) (ie, ferumoxide [Endorem], and ferucarbotran [Resovist]). These substances are transported out of the blood circulation and taken up by the reticuloendothelial system (or Kupffer cells of the liver) through phagocytosis. These particles cause magnetic field inhomogeneities with shortening of T2 relaxation time, leading to a decrease in the signal of the normal liver parenchyma on T2-weighted sequences, best seen on T2 or GRE sequences for lesion detection. However, for lesion characterization T2-weighted turbo spin-echo (TSE) sequences or HASTE (half-Fourier acquisition single-shot turbo spin-echo) sequences are more sensitive for evaluating signal intensity loss. Depending on the content of the Kupffer cells, benign hepatocellular lesions show uptake of these contrast agents. Hemangiomas present an exception for SPIO contrast media uptake because of their relatively large, slow-flowing blood pool that allows the effect of the SPIO particles to occur and therefore, although lacking Kupffer cells, show a decrease in signal on T2-weighted images.
Lesions that do not contain Kupffer cells (eg, malignant lesions, metastases) do not take up SPIO contrast agents, rendering them bright against the background of the dark liver parenchyma on T2-weighted post-SPIO sequences. The resulting increase in contrast between the liver and a lesion of nonhepatic origin increases the detection rate compared with unenhanced images and allows an easy distinction from benign hepatic lesions. Unfortunately, one of these agents (ferucarbotran) will no longer be available after the beginning of 2011.
The second group of liver-specific contrast agents includes the hepatobiliary contrast media, which are selectively taken up by hepatocytes and excreted through the biliary tracts. This activity results in an increase in signal of the normal liver parenchyma on T1-weighted sequences. Malignant lesions show washout, appearing hypointense, and thus become more conspicuous. Mangafodipir (Mn-DPDP), gadobenate dimeglumine (Gd-BOPTA), and gadoxetate (Gd-EOB-DTPA) belong to this group of contrast media. The manganese-based contrast agent Mn-DPDP (formerly known as mangafodipir trisodium or teslascan ) is a contrast medium that enhances the liver parenchyma and bile ducts through this pathway of excretion on T1-weighted sequences. This contrast agent was unfortunately discontinued recently. The gadolinium-based bimodal contrast agents show combined perfusion- and hepatocyte-specific properties. The subgroup of gadolinium-based contrast material comprises gadobenate dimeglumine (MultiHance, Bracco Diagnostics, Princeton, NJ, USA) and gadoxetate (Primovist, Bayer Schering Pharma, Berlin, Germany). These substances are administered intravenously as a bolus for dynamic imaging in the arterial, portal venous, and delayed phases, allowing the evaluation of morphology, vascularity, and functional properties within a single examination.
Cysts
Hepatic cysts are the most common benign focal liver lesions, with an incidence of 2% to 7%. They can be further differentiated into simple cysts, cysts in autosomal dominant polycystic kidney disease, ciliated hepatic foregut cysts, and parasitic (hydatid) cysts.
Simple Cysts
Simple cysts are well-defined, round, or oval lesions. They are lined with very thin or imperceptible layers of fibrous tissue and show no communication to the biliary tree. The origin of simple hepatic cysts is unclear, but developmental and acquired causes that lead to the retention of bile are postulated. On MR imaging, these cysts show homogenous low signal intensity on T1-weighted images. In the rare condition that these cysts become hemorrhagic or contain protein, they show an increase in signal intensity on T1-weighted images. Because of the very long T2-time of fluid, cysts retain signal on sequences with long echo times (eg, >120 ms), showing very high signal intensity on T2-weighted images.
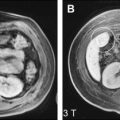
In autosomal dominant polycystic kidney disease, hepatic cysts are found in 40% of the cases, with the liver the primary site for extrarenal cyst manifestation. These cysts tend to be multiple and varied in size (usually <2 cm) ( Fig. 1 ). Intercystic hemorrhage is rare but is encountered more often than in simple hepatic cysts without this underlying disease.
Ciliated Hepatic Foregut Cyst
Ciliated hepatic foregut cysts are very rare benign congenital solitary lesions believed to arise from the embryonic foregut. They are usually unilocular, smaller than 3 cm, and are most often situated in the left liver lobe at the anterosuperior liver margin or intersegmentally, where they tend to bulge the outer contour of the liver. The content can show various viscosities, from serous to mucinous, which affects the MR signal intensity, especially in T1-weighted images. The signal intensity can be low or high or anywhere in between. On T2-weighted imaging, the cyst content is homogenously hyperintense ( Fig. 2 A ).
Using DWI, simple and ciliated hepatic foregut cysts and liver cysts in polycystic kidney disease show a signal intensity decrease with increasing b-values, and are strongly hyperintense and homogeneous on analog-to-digital (ADC) sequences ( Fig. 2 B–D). After application of contrast material, either gadolinium chelate or liver-specific contrast agents, the fluid content of hepatic cysts never shows uptake of contrast material, this being a useful feature in differentiating hepatic cysts from poorly vascularized malignant cystic lesions ( Fig. 2 E).
Echinococcal Cysts
Echinococcus granulosus is a parasite that causes hydatid cysts, predominantly in the liver and lung and, less commonly, the heart and spleen. Hydatid cysts are usually round in shape, have a fibrous capsule, and are unilocular or multiseptated. Because of the frequent presence of smaller daughter cysts within the periphery of the mother cyst, the hydatid cyst often appears multicystic. The fluid component of the hydatid cyst is typically low on T1-weighted and high on T2-weighted images. In the presence of interluminal debris, the signal intensity can become moderately inhomogeneous on both T1- and T2-weighted images. Degenerated cysts decrease in size and appear heterogeneous, solid, or pseudo-tumor-like, whereas dead cysts are characterized by a thickened calcified wall. The fibrous capsule and internal septa appear hypointense on T2-weighted images and show enhancement in the post-gadolinium phase ( Fig. 3 ); however, these structures are better visualized on ultrasound than MR imaging or CT, whereas capsular calcifications are better depicted with CT than with MR imaging.
The parasite E multilocularis produces multilocular alveolar cysts in any body organ or tissue, but the liver is the site most commonly affected, which leads to hepatic alveolar echinococcosis (HAE). HAE cysts are, in contrast to hydatid cysts, not true cysts. HAE cysts are solitary, multilocular, or confluent; 1 to 10 mm in diameter; resemble alveoli; and can grow to between 15 and 20 cm. HAE cysts show irregular margins, lack a fibrous capsule, and have cystic and solid components (coagulation necrosis, granuloma, and calcification). HAE cysts tend to involve extensive regions of the liver, with a propensity for the porta hepatis, causing stenoses of bile ducts, portal, and hepatic veins, and can lead to portal hypertension. These lesions are hypointense on T1- weighted images and can be hypo-, iso-, or hyperintense on T2-weighted imaging ( Fig. 4 ). In post-gadolinium images, these lesions appear inhomogeneous. As the lesion heals, it begins to calcify, first in a scattered form, and eventually becomes a large calcified mass. Again, calcification is better depicted with CT. DWI of hydatid cysts shows the same characteristics as the simple cysts and hepatic foregut cysts. The appearance of HAE cysts in DWI is inhomogeneous when calcifications are present.
Biliary hamartoma/bile duct hamartoma
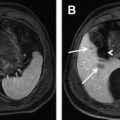
Bile duct hamartomas, also known as biliary microharmatomas or von Meyenburg complexes , are rare, benign, cystic lesions that are thought to arise from ductal plate malformations of the small interlobular bile ducts. At histopathology, these lesions appear as a collection of small, sometimes dilated, irregular and branching bile ducts, embedded in a fibrous stoma. They are usually less than 1 cm in diameter, can be numerous, and are usually incidental radiologic findings. Bile duct hamartomas are well-defined lesions that show low signal intensity on T1-weighted imaging and appear strongly hyperintense on T2-weighted imaging ( Fig. 5 A ). On post-gadolinium images, these cystic lesions show no internal enhancement; however, thin rim enhancement on early and late post-gadolinium images is typical ( Fig. 5 B). The enhancing rim consists of compressed adjacent hepatic parenchyma and should not be mistaken for a fibrous capsule. This rim enhancement may lead to the misinterpretation of a metastatic lesion; however, biliary hamartomas, as opposed to metastases, do not show perilesional contrast enhancement or progressive centripetal fill-in on equilibrium-phase after administration of gadolinium contrast. Biliary hamartomas also do not show uptake of liver-specific contrast agents, because these lesions do not communicate with the bile ducts. On DWI, biliary hamartomas show the typical features of cystic lesions because of the significant signal intensity loss with increasing b-values ( Fig. 5 C, D), and appear strongly hyperintense on the ADC sequences.
Hemangioma
Hemangioma is the most common benign liver neoplasm, with a prevalence of up to 20%. It is usually an incidental finding in patients at any age, but is up to five times more common in women, and can be found as a solitary lesion or as multiple lesions. Hemangiomas are thought to be hamartomatous lesions and are usually well contained within the liver. On rare occasions, however, hemangiomas can cause a bulge of the liver surface, showing exophytic or even extrahepatic growth, with only a thin stalk connecting them to the liver, as in a pedunculated hemangioma ( Fig. 6 ). However, this has no effect on the appearance on unenhanced MR images or the contrast enhancement pattern. On rare occasions, hemangiomas can become symptomatic when, because of large size, they cause compression of adjacent structures, and in 1% to 4% of cases may rupture and bleed into the peritoneum. A pedunculated hemangioma may undergo torsion and infarction.
At histopathology, hemangiomas appear as multiple vascular channels lined by a single layer of endothelial cells, and can be classified into two predominant types according to the size of their vascular spaces. The most common is the cavernous hemangioma, consisting of numerous large vascular channels separated by thin fibrous septae. The second most common is the capillary hemangioma (16%), which differs from the cavernous type by the presence of narrower vascular spaces with regard to the numerous capillary channels. Hemangiomas can also be classified according to their size. A capillary hemangioma has a size ranging from 1 to 2 cm, whereas the cavernous hemangioma can measure up to 5 cm, and a giant hemangioma is classified as a lesion greater than 5 cm in diameter. At unenhanced MR imaging, hemangiomas are seen as well-delineated lesions that are round in shape when small and tend to show lobular borders when larger. Because of their very long T2 time, hemangiomas retain signal on heavily T2-weighted images (echo time>120 ms), consequently appearing homogeneously hyperintense, and conversely, showing low signal intensity on T1-weighted MR images ( Fig. 7 ). On T2-weighted images, giant hemangiomas frequently show a central area of either bright, dark, or mixed signal intensity and a network of multiple fibrous septae of low signal intensity (see Fig. 6 ). At histopathology, the bright central area corresponds to hypocellular myxoid tissue. Furthermore, hemorrhage, small areas of thrombosis, calcification, and areas of extensive fibrosis can rarely be seen in giant hemangiomas. Depending on the uptake of contrast material, hemangiomas show three typical enhancement patterns on post-gadolinium dynamic images.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree