MR neurography can aid diagnosis and management of diffuse peripheral neuropathies. Diffuse peripheral neuropathies can be due to a variety of causes, including hereditary, inflammatory/autoimmune, infectious, and secondary to systemic conditions, such as diabetes mellitus. MR findings include long-segment (typically more than 6 cm) nerve enlargement and signal hyperintensity and may involve a solitary nerve or multiple nerves, depending on the underlying condition. Importantly, in the interpretation of diffusely abnormal nerves by imaging, neoplastic cause should be ruled out.
Key points
- •
Diffuse peripheral neuropathy can be diagnostically challenging owing to the involvement of multiple nerves and the wide range of pathologic conditions that can lead to diffuse peripheral nerve abnormalities on imaging.
- •
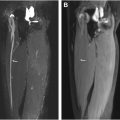
Diffuse peripheral nerve lesions are categorized as either solitary neuropathy or polyneuropathy and entail long-segment involvement, which is defined as greater than 6 to 12 cm.
- •
MR neurography can localize and characterize nerve pathologic condition and help differentiate between different causes of peripheral neuropathy.
| ADC | apparent diffusion coefficient |
| AIDP | acute inflammatory idiopathic polyneuropathy |
| CIP | critical illness neuropathy |
| CIPD | chronic inflammatory idiopathic polyneuropathy |
| CMT | Charcot-Marie-Tooth syndrome |
| DA | diabetic amyotrophy |
| FS | fat-suppressed |
| MIP | maximum intensity projection |
| MMN | multifocal motor neuropathy |
| MPR | multiplanar reformation |
| MRN | magnetic resonance neurography |
| POEMS | polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, skin changes |
| STIR | short tau inversion recovery |
| VEGF | vascular endothelial growth factor |
| WB | whole body |
Introduction
Peripheral neuropathy can be a debilitating condition, with overall prevalence of 1% in the general population and 7% in the elderly. Symptoms include pain, weakness, loss of function, and in some cases, end-organ damage with muscle atrophy. Although the clinical examination and electrodiagnostic studies are traditional methods of assessing peripheral neuropathy, imaging is playing an increasingly important role in the diagnosis of peripheral nerve disorders. The distribution of peripheral neuropathy as focal or multifocal, symmetric or asymmetric, and proximal or distal is a helpful feature in determining the underlying cause. MR imaging of peripheral nerves, also known as magnetic resonance neurography (MRN), allows for visualization of nerve architecture, course, and signal. MRN can localize and characterize nerve pathologic condition and help differentiate between different causes of peripheral neuropathy with high diagnostic accuracy. ,
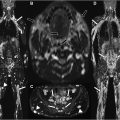
In the recently described Neuropathy Score Reporting and Data System, diffuse peripheral nerve lesions were defined as diffuse long-segment nerve hyperintensity on T2-weighted imaging or enlargement ( Fig. 1 ), with “long-segment” involvement being defined as greater than 6 to 12 cm. Diffuse peripheral nerve lesions were categorized as either diffuse solitary/mononeuropathy (D1, affecting a single nerve) or diffuse polyneuropathy (D2, affecting multiple nerves, either unilateral or bilateral). , There is otherwise limited prior literature on the utility of MRN in diffuse peripheral nerve lesions. Long-segmental involvement of single or multiple nerves on MR imaging can be diagnostically challenging owing to the nonspecific appearance and the wide range of pathologic conditions that can lead to diffuse peripheral nerve lesions ( Table 1 ).

| Cause | Most Common Cause |
|---|---|
| Immune-mediated | Acute inflammatory idiopathic polyneuropathy (AIDP), chronic inflammatory idiopathic polyneuropathy (CIDP), multifocal motor neuropathy (MMN), neuralgic amyotrophy/brachial plexitis |
| Infectious | Leprosy, Epstein-Barr virus, Herpes Zoster virus, long-standing HIV, and B burgdorferi (Lyme disease) |
| Hereditary | Charcot-Marie-Tooth types 1A, 1B, and X-linked |
| Systemic disease–related | Diabetes mellitus, long-standing HIV infection, critical illness, end-stage kidney disease/uremic, amyloidosis, hypothyroidism, vitamin deficiency |
| Toxic | Alcohol, chemotherapeutic agents, most heavy metals, many commonly used medications |
| Environmental | Vibration-induced, prolonged cold exposure, hypoxemia |
| Neoplastic/paraneoplastic | Secondary nonneurogenic tumors due to contiguous spread, hematologic malignancy, POEMS syndrome |
| Idiopathic | No specific cause |
Diffuse peripheral mononeuropathies can be caused by neoplasms or a wide variety of entities, such as infection, ischemia, trauma including traction nerve injury, idiopathic entities, such as hypertrophic mononeuropathy, and systemic disorders. Diffuse peripheral polyneuropathies can also be neoplastic in cause and otherwise be autoimmune, hereditary, toxin-related, systemic disease-related, or idiopathic. The purpose of this review article is to describe the key clinical and imaging features of diffuse peripheral mononeuropathy or polyneuropathy that can aid the radiologist in arriving at an accurate diagnosis or generate a succinct and relevant differential diagnosis.
MR imaging features of diffuse peripheral neuropathy
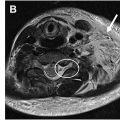
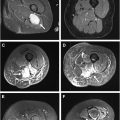
MR imaging findings of diffuse peripheral neuropathy include long-segment T2 signal hyperintensity and/or enlargement of a single nerve or multiple nerves. , The degree of signal hyperintensity and enlargement, the number of nerves affected, the distribution, and the pattern of abnormal findings vary based on the cause of diffuse peripheral neuropathy. Intravenous contrast administration is not typically necessary for the assessment of diffuse peripheral neuropathy, although contrast enhancement can be a supportive feature for the diagnosis of neoplastic cause, noting that contrast enhancement can also be a feature of nonneoplastic pathologic condition. MR imaging findings can complement clinical history, physical examination, and laboratory analysis to aid in diagnosis of diffuse peripheral neuropathy, assess for treatment response, and guide management decisions. A discussion of imaging features found with different causes is discussed in further detail in later discussion and is summarized in Table 2 .
| Cause | Clinical Characteristics | MR Imaging Features |
|---|---|---|
| Acute inflammatory idiopathic polyneuropathy | Symmetric polyneuropathy with lower-extremity weakness or flaccid paralysis that progresses to upper extremities Antecedent event, such as infection Peak symptoms within 3–4 wk Albuminocytologic disassociation on LP Decreased or absent DTRs | Thickening and enhancement of intraspinal nerve roots; cauda equina and conus medullaris |
| Chronic inflammatory idiopathic polyneuropathy | Symmetric polyneuropathy characterized by proximal and distal sensorimotor involvement Gradually progressive over several months Albuminocytologic disassociation on LP Decreased or absent DTRs | Symmetric (> asymmetric) thickening and enhancement of peripheral nerves, brachial and lumbosacral plexus and nerve roots Facilitated diffusion on DWI |
| Multifocal motor neuropathy | No objective sensory abnormalities Upper-limb motor deficit Decreased/absent DTRs in affected limb | Asymmetric thickening of the affected upper-extremity motor peripheral nerve |
| Neuralgic amyotrophy | Abrupt onset of severe pain followed by patchy weakness in the distribution of the upper and/or middle brachial plexus ± Winged scapula | Asymmetric focal or multifocal thickening of the brachial plexus or upper extremity/chest wall peripheral nerve or nerves ± hourglass constrictions |
| Charcot-Marie-Tooth | Symmetric polyneuropathy Distal weakness and atrophy Foot drop Pes cavus Family history | Symmetric masslike thickening of the peripheral nerves, brachial and lumbosacral plexus, and nerve roots with variable to no enhancement Facilitated diffusion on DWI |
| Diabetic amyotrophy | Asymmetric polyneuropathy Acute onset of unilateral leg pain followed by progressive weakness that involves the contralateral leg | Asymmetric thickening of lumbar plexus or lower-extremity peripheral nerve |
| Amyloidosis | Mixed sensory and motor peripheral neuropathy ± autonomic dysfunction | Maybe asymmetric or symmetric multifocal peripheral nerve/plexus enlargement, propensity for carpal tunnel syndrome |
| Critical illness polyneuropathy | Severe sepsis and multiorgan failure Failure to wean from the ventilator COVID-19 infection Decreased or absent DTRs | Asymmetric thickening of upper- and/or lower-extremity peripheral nerves/plexi |
| Radiation-induced neuropathy/plexopathy | Antecedent history of malignancy treated with radiation | Asymmetric thickening with variable “tram-track” enhancement concordant with the radiation field Facilitated diffusion on DWI |
| POEMS syndrome | Organomegaly, endocrinopathy, monoclonal plasma cell disorder, skin changes Symmetric distal painful (sensory before motor) and progressive, proximal spread | Symmetric thickening and enhancement of peripheral nerves, brachial and lumbosacral plexus, and nerve roots |
| Malignant peripheral neuropathy/plexopathy | History of metastatic disease/hematologic malignancy Painful neuropathy Hypermetabolic activity corresponding to the plexus or peripheral nerve on PET imaging | Asymmetric masslike thickening and enhancement of plexus or peripheral nerve Adjacent or contiguous neoplasm Restricted diffusion on DWI |
Immune-mediated neuropathies
Autoimmune processes can affect peripheral nerves and result in both acute and chronic peripheral neuropathy. Acute autoimmune neuropathy typically presents with sudden-onset pain, weakness, and paresthesia and has been associated with infection, trauma, and surgery.
Acute Inflammatory Idiopathic Polyneuropathy
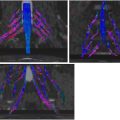
Acute inflammatory idiopathic polyneuropathy (AIDP), or Guillain-Barre syndrome, results in acute progressive relatively symmetric motor and sensory symptoms with associated diminished or absent deep tendon reflexes. Lumbar puncture often shows albuminocytologic dissociation (elevated protein and normal to mildly elevated leukocyte count [usually <5 cells/mm 3 ]). Typically, the diagnosis of AIDP is made on a combination of clinical features and cerebrospinal fluid analysis. In atypical clinical presentations or equivocal lumbar puncture, imaging can be performed to support the diagnosis and exclude other causes of polyneuropathy. MR findings of AIDP ( Fig. 2 ) include thickening and enhancement of the anterior more than posterior spinal nerve roots, particularly the cauda equina and conus medullaris. In subtle cases, thickening of the intraspinal nerve roots may be challenging to detect, and the administration of intravenous contrast material may be useful. Treatment is conservative, and recovery is expected over time, generally 4 to 6 weeks. MRN is helpful for diagnosis and to exclude other potential causes of the patient’s symptoms.