Ductal carcinoma in situ (DCIS) now accounts for well over 20% of cancers diagnosed in mammography screening programs. This article reviews the biology of DCIS and the unique kinetic and morphologic features of DCIS at MR imaging that allow reliable diagnosis of DCIS lesions, including noninvasive disease that is mammographically occult. Increased detection of DCIS lesions at MR imaging has important implications for high-risk screening programs and for staging of patients with newly diagnosed breast cancer. Future research may provide prognostic information extracted from the MR phenotype of in situ cancer.
Detection of ductal carcinoma in situ (DCIS) has increased dramatically since the advent of widespread screening for breast cancer in the early 1980s. DCIS is a preinvasive form of breast cancer whereby a clonal proliferation of malignant epithelial cells, originating in the terminal ductal lobular unit, remain confined to ducts and do not invade beyond the basement membrane into the surrounding breast tissue. Evidence suggests that approximately 30% to 50% of DCIS lesions will progress to become invasive. DCIS may involve multiple sites separated by normal tissue contained within the same ductal system (“skip lesions”) or in adjacent or remote ductal systems. DCIS, rarely diagnosed before the advent of mammographic screening, now accounts for well over 20% of malignant lesions found in screen-detected series.
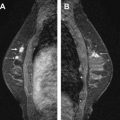
Early reports in the 1930s of a precursor lesion that could progress to invasion led to first use of the term “carcinoma in situ.” In the era before screening, most in situ lesions were detected clinically as a palpable mass, Paget disease, or nipple discharge. Nowadays, DCIS typically presents as a nonpalpable, 10- to 20-mm calcified lesion discovered on mammography. Calcifications predictive of DCIS are visible in up to 90% of DCIS cases diagnosed on mammography alone, usually exhibiting a clustered, linear, or segmental distribution. The typical fine linear calcifications visible at mammography are probably caused by necrosis secondary to hypoxia occurring centrally in DCIS lesions, as blood supply by diffusion from extraductal vessels becomes inadequate due to tumor growth. Although calcified necrosis is a frequent mammographic finding in DCIS, the presenting finding may be a mass, asymmetry, or architectural distortion in approximately 10% to 20% of cases.
The increase in rates of DCIS diagnosis in the United States is strongly associated with the concurrent increase in rates of mammography screening, and is similar to the increasing incidence of DCIS in other developed countries where breast screening programs are conducted. DCIS incidence in the United States increased sevenfold, from 1973 through the late 1990s, with the most rapid increases found among women older than 50 years. The current age-adjusted incidence rate of DCIS in the United States is 32.5 per 100,000 women; at ages 50 to 64 years, the incidence is approximately 88 per 100,000. As of January 1, 2005, an estimated 500,000 United States women were living with a diagnosis of DCIS, and this number is estimated to increase to more than 1 million women by 2020, assuming constant incidence and survival rates. The American Cancer Society estimates that carcinoma in situ will account for 24.5% (n = 62,280) of all breast cancer cases diagnosed in the United States in 2009. Few studies have focused on risk factors for DCIS; however, it is generally agreed that the risk factors are the same as those for invasive breast cancer. These indicators include increasing age, family history of breast cancer, genetic predisposition, high mammographic density, late age at menopause, nulliparity, menopausal estrogen with progestin therapy, late age at first birth, and high postmenopausal body mass index. Mammography has proved to be uniquely successful in the identification of preinvasive cancer, resulting in highly favorable patient outcomes.
Biology of DCIS
The natural history of DCIS, and of breast cancer overall, is still poorly understood. Noninvasive cancers comprise a heterogeneous group of lesions with variable biologic, genetic, and histopathologic features, and are detected typically on x-ray mammography as microcalcifications formed inside necrotic tumors within the ducts. Tumor characteristics of in situ lesions at histology involve assessment of both qualitative and quantitative features. The qualitative features of DCIS include assessment of the architectural growth pattern, nuclear grade (high-, intermediate-, and low-grade cytologic features), and presence or absence of central necrosis. The most aggressive form of DCIS, the type that is most likely to progress to invasion, is associated with high-grade cellular and nuclear features and central necrosis “comedo type,” and is associated with microcalcifications contained within the ducts. In fact, “comedo” cancer in the 1950s and 1960s was invariably classified as an invasive cancer, because it was always diagnosed late by physical findings. Other architectural types are classified as cribriform, papillary, micropapillary, and solid types, with mixed histologic subtypes found in more than one-half of DCIS cases.
Nuclear grading and histologic growth patterns of in situ lesions have been associated with morphologic characteristics of microcalcifications seen at mammography. There has been recent interest in the possibility that the mammographic phenotypes of breast lesions may convey important prognostic indicators or biomarkers. Women in screening mammography trials, with linear/linear-branching, or “casting” type of calcifications, have been shown to have considerably poorer survival outcome than women with amorphous or punctate calcification morphology. Tabar and colleagues have suggested that “casting” calcifications at mammography represent a “duct-forming“ invasive cancer. DCIS lesions are considered to be nonobligate precursors of invasive cancer, and when progression to invasion occurs, the invasive cancers exhibit similar cytologic features, expression profiles, receptor status, and genomic characteristics. Investigations into genetic, biologic, and histopathological features of DCIS have shown many possible expressions of biochemical markers such as progesterone and estrogen receptors, Ki-67, PCN, c-erbl, and Cathepsin E. The molecular profiles of DCIS and invasive breast cancer are similar, supporting a common origin. In general, high-grade noninvasive lesions exhibit rapid growth rates and high mitotic indices, and will almost invariably progress to an invasive cancer, whereas low-grade lesions may remain quiescent, never invade, and if they do will progress to low-grade invasive cancer.
With increasing numbers of small cancers detected at mammography screening, breast conservation therapy is widely performed, underscoring the importance of accurate depiction of the extent of DCIS for optimal cancer staging and surgical planning. Measures of mammographic microcalcifications have been shown to significantly underestimate DCIS extent. Holland and colleagues reported that discrepancies found between mammographic and histologic estimations of the extent of noninvasive cancer were related to the histologic type of DCIS, with high-grade lesion extent at mammography, more closely correlated with histologic measurements than estimates for low grade lesions. In that study, mammographically occult DCIS was present in 16% of comedo-type DCIS lesions larger than 20 mm, whereas occult disease was present in 47% of predominantly micropapillary-cribriform growth types. Standardization systems for the histopathologic classification of DCIS have been proposed by several investigators, notably the scheme introduced by Holland and colleagues and the system known as the Van Nuys classification. The Van Nuys system stratifies DCIS patients into three groups by combining high nuclear grade and “comedo-type” necrosis. In this system, low nuclear grade cases lacking comedo-type necrosis are placed into group 1, and into group 2 if necrosis is present, with all high nuclear grade cases defined as group 3. Although mammography still remains an important method for diagnosing DCIS, it is clear that many in situ lesions may be partially or completely occult at mammography. Thus, women who undergo surgical excision of DCIS lesions diagnosed only at mammography may experience an inadequate surgical resection, with margins positive for in situ cancer, resulting in the need for reexcision surgery.
Against the background of extensive study of DCIS at mammography, including careful mammographic/histopathologic correlative investigations, additional imaging methods aimed to improve DCIS detection were sought, particularly with a view to improving the estimation of extent of disease in women with newly diagnosed breast cancer. Accurate depiction of the extent of DCIS, with or without the presence of an invasive component, is now considered essential for successful breast conservation therapy, although the original National Surgical Adjuvant Breast and Bowel Project (NSABBP) trials did not require negative margins. Noninvasive carcinoma with negative margins achieved at breast-conserving surgery results in a recurrence rate of 22.5% when treated without radiation therapy. This rate increases if close or positive margins are present at surgical resection. Whole-breast radiation therapy has been shown to reduce the recurrence rate by 50%. Treatment of estrogen receptor–positive cases with tamoxifen reduces this risk by an additional 50%. However, it is important that regardless of the therapeutic protocol (mastectomy, lumpectomy with radiation therapy, or wide surgical excision alone), half of the DCIS lesions are invasive when they recur, and 20% of cases present with distant metastases at 10 years. Improved identification of DCIS, especially in high-risk women, could result in better patient outcome. Accordingly, further improvement in the accuracy of diagnosis of DCIS is an important goal, particularly because early diagnosis at a preinvasive stage yields excellent survival with local treatment only (surgical excision with or without radiotherapy).
MR imaging characteristics of DCIS
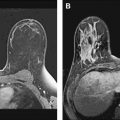
Dynamic contrast-enhanced MR imaging (DCE-MRI) has been shown to visualize invasive breast cancer reliably; however, detection of in situ cancer has proved more difficult, with disappointing early reports indicating relatively low diagnostic accuracy and a variable reported sensitivity and specificity for DCIS. Initial MR imaging screening trials reporting DCIS cases identified by mammography but occult to MR imaging supported a limited role for MR imaging in the detection of noninvasive disease. DCE-MRI allows cancer to be distinguished from normal tissue because of the increased vascularity and capillary permeability of malignant lesions. More recent reports suggest that DCE-MRI performs as well as or better than x-ray mammography in the task of identifying DCIS lesions. A study of 127 women with pure DCIS, reported in 2007 by Kuhl and colleagues, reported that the sensitivity of MR imaging was vastly superior to mammography, 92% versus 56%, in the detection of DCIS. This study also showed a strong MR imaging sensitivity for high-grade lesions, 98%, compared with only 52% sensitivity for mammography. The majority of cases in this study not detected at MR imaging were low-grade lesions (87%). The improvement in DCIS detection in recent studies is likely due to improved spatial and temporal resolution made possible with modern DCE-MRI methods.
Studies have shown that enhancement kinetics, that is, the time course of the signal intensity within the lesion, can be used as an additive factor to morphology in the determination of the likelihood of malignancy for enhancing lesions visible at MR imaging. Multiple reports over the last 20 years have culminated in reproducible, routine morphologic and kinetic standards, used today in clinical practice, to distinguish benign from malignant breast lesions. Improvements in the accuracy of kinetic analysis are still needed, however, as many benign and malignant lesions exhibit considerable kinetic overlap.
The BI-RADS lexicon describes 3 types of enhancing lesions seen on breast MR imaging:
- 1.
Focus , defined as a spot of enhancement that is too small (<5 mm) to allow confident further characterization
- 2.
Mass , defined as a 3-dimensional space-occupying lesion
- 3.
Nonmass-like enhancement (NMLE) , defined as enhancement of an area that is not a mass.
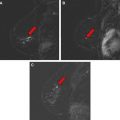
Although less common, NMLE is the predominant morphology pattern found in DCIS. Moreover, these lesions exhibit variable kinetic curve shapes, and may be more difficult to detect at MR imaging. Kinetic time course curves reflect perfusion and diffusion of contrast media, from blood vessels to the extracellular space, and are related to the unique physiology and vasculature of invasive, benign, and pure DCIS lesions. In a recent pilot study based on a relatively small number of patients, Jansen and colleagues used an empirical mathematical model to analyze kinetic curves in NMLE versus mass lesions, and found that NMLE lesions exhibited significantly lower contrast uptake and slower washout compared with mass lesions. Furthermore, sensitivity and specificity of kinetic analysis was reduced in NMLE lesions compared with mass lesions. More specifically, it is likely that the kinetic parameters and criteria that work best to distinguish benign and malignant mass lesions may not work well with nonmass lesions, and vice versa. Others have pointed out that the considerable overlap of kinetic patterns of DCIS with benign lesions compromises its reliable identification.
DCIS lesions often exhibit kinetic curves that persistently rise or plateau over time, a pattern not often seen with invasive malignancies. Of note, most prior studies of the kinetics of DCIS have used clinically acquired DCE-MRI data with relatively low temporal (45–90 seconds) resolution to allow for 3-dimensional whole breast imaging, and have studied contrast kinetics in one small region of interest (ROI) placed within the lesion. The kinetic curves of NMLE lesions are vulnerable to partial volume effects, as small ROIs encompassing part of the lesion also capture some of the surrounding normal tissue, diluting the reliability of the acquired enhancement data. It is understandable, therefore, that the observed differences between mass and NMLE lesions at MR imaging may be accentuated due to these partial volume effects. Although relatively sparse temporal resolution (ie, 3–6 postcontrast images) is typical for clinical breast DCE-MRI, it could be that imaging at much higher temporal resolution, with a greater number of time points, would aid substantially in lesion characterization.
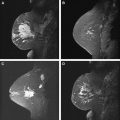
Reports correlating in situ lesions and their relationship to nuclear grade enhancement characteristics at MR imaging and mammographic findings are rare. Jansen and colleagues performed a qualitative and quantitative evaluation of 82 consecutively diagnosed pure DCIS lesions on DCE-MRI and classified results according to nuclear grade and x-ray mammographic presentation. In this study, the lesion population was segmented into 4 groups according to their mammographic presentation:
- 1.
Fine pleomorphic, fine linear, or fine linear-branching calcifications (32 of 63)
- 2.
Amorphous or indistinct calcifications (14 of 63)
- 3.
Noncalcified masses (8 of 63)
- 4.
Mammographically occult cases (9 of 63).
Significant differences in the kinetic behavior of these populations were found.
Lesions with fine pleomorphic, fine linear, or fine linear-branching calcifications, and especially those presenting as masses on x-ray mammography, were more suspicious by conventional kinetic standards on MR imaging than lesions with amorphous or indistinct calcifications. In particular, lesions appearing as masses on mammography exhibited washout curves on average, whereas calcifications and occult lesions exhibited plateau curves. Nonmass, clumped enhancement in a segmental or linear distribution predominated. Pure DCIS lesions demonstrated 28% persistent, 27% plateau, and 45% washout behavior, which is similar to distributions found in other reports. For pure DCIS lesions, the average enhancement at 1 minute (E 1 ) was 201%. The reported time to peak enhancement (T peak ) for invasive and benign lesions was 230 seconds, implying that pure DCIS lesions enhance less than invasive cancers and more than benign lesions.
It is interesting that the percent enhancement parameters (E 1 and E peak ) were statistically equivalent across all groups by mammographic and nuclear grade stratification. In this report, the signal enhancement ratio (SER) values were statistically equivalent for pure DCIS lesions of various grades; however, SER values varied by mammographic presentation. This finding suggests that washout as measured by SER may be a more important diagnostic parameter for assessment of in situ lesions than percent enhancement. No significant difference in enhancement kinetics among different nuclear grades of pure DCIS was found.
The findings in this article support previous reports by Viehweg and colleagues, and suggest that x-ray mammographic appearance of pure DCIS may be related to its underlying physiology and vasculature in a way that nuclear grading is not. Additional reports have indicated that perfusion rates increase from benign to DCIS to invasive cancers and are correlated with microvessel density in DCIS lesions. Guidi and colleagues showed an increase in vessel density around ducts with DCIS, although with variable patterns. Heffelfinger and colleagues found that the expression of angiogenic growth factors such as vascular endothelial growth factor increases from hyperplasia to DCIS. Esserman and colleagues studied the relationship between SER values of invasive tumors and tumor vascularity and histologic grade, and found that higher SER values correlate with higher vascularity and higher Scarff-Bloom-Richardson (SBR) pathologic grade.
MR imaging characteristics of DCIS
Dynamic contrast-enhanced MR imaging (DCE-MRI) has been shown to visualize invasive breast cancer reliably; however, detection of in situ cancer has proved more difficult, with disappointing early reports indicating relatively low diagnostic accuracy and a variable reported sensitivity and specificity for DCIS. Initial MR imaging screening trials reporting DCIS cases identified by mammography but occult to MR imaging supported a limited role for MR imaging in the detection of noninvasive disease. DCE-MRI allows cancer to be distinguished from normal tissue because of the increased vascularity and capillary permeability of malignant lesions. More recent reports suggest that DCE-MRI performs as well as or better than x-ray mammography in the task of identifying DCIS lesions. A study of 127 women with pure DCIS, reported in 2007 by Kuhl and colleagues, reported that the sensitivity of MR imaging was vastly superior to mammography, 92% versus 56%, in the detection of DCIS. This study also showed a strong MR imaging sensitivity for high-grade lesions, 98%, compared with only 52% sensitivity for mammography. The majority of cases in this study not detected at MR imaging were low-grade lesions (87%). The improvement in DCIS detection in recent studies is likely due to improved spatial and temporal resolution made possible with modern DCE-MRI methods.
Studies have shown that enhancement kinetics, that is, the time course of the signal intensity within the lesion, can be used as an additive factor to morphology in the determination of the likelihood of malignancy for enhancing lesions visible at MR imaging. Multiple reports over the last 20 years have culminated in reproducible, routine morphologic and kinetic standards, used today in clinical practice, to distinguish benign from malignant breast lesions. Improvements in the accuracy of kinetic analysis are still needed, however, as many benign and malignant lesions exhibit considerable kinetic overlap.
The BI-RADS lexicon describes 3 types of enhancing lesions seen on breast MR imaging:
- 1.
Focus , defined as a spot of enhancement that is too small (<5 mm) to allow confident further characterization
- 2.
Mass , defined as a 3-dimensional space-occupying lesion
- 3.
Nonmass-like enhancement (NMLE) , defined as enhancement of an area that is not a mass.
Although less common, NMLE is the predominant morphology pattern found in DCIS. Moreover, these lesions exhibit variable kinetic curve shapes, and may be more difficult to detect at MR imaging. Kinetic time course curves reflect perfusion and diffusion of contrast media, from blood vessels to the extracellular space, and are related to the unique physiology and vasculature of invasive, benign, and pure DCIS lesions. In a recent pilot study based on a relatively small number of patients, Jansen and colleagues used an empirical mathematical model to analyze kinetic curves in NMLE versus mass lesions, and found that NMLE lesions exhibited significantly lower contrast uptake and slower washout compared with mass lesions. Furthermore, sensitivity and specificity of kinetic analysis was reduced in NMLE lesions compared with mass lesions. More specifically, it is likely that the kinetic parameters and criteria that work best to distinguish benign and malignant mass lesions may not work well with nonmass lesions, and vice versa. Others have pointed out that the considerable overlap of kinetic patterns of DCIS with benign lesions compromises its reliable identification.
DCIS lesions often exhibit kinetic curves that persistently rise or plateau over time, a pattern not often seen with invasive malignancies. Of note, most prior studies of the kinetics of DCIS have used clinically acquired DCE-MRI data with relatively low temporal (45–90 seconds) resolution to allow for 3-dimensional whole breast imaging, and have studied contrast kinetics in one small region of interest (ROI) placed within the lesion. The kinetic curves of NMLE lesions are vulnerable to partial volume effects, as small ROIs encompassing part of the lesion also capture some of the surrounding normal tissue, diluting the reliability of the acquired enhancement data. It is understandable, therefore, that the observed differences between mass and NMLE lesions at MR imaging may be accentuated due to these partial volume effects. Although relatively sparse temporal resolution (ie, 3–6 postcontrast images) is typical for clinical breast DCE-MRI, it could be that imaging at much higher temporal resolution, with a greater number of time points, would aid substantially in lesion characterization.
Reports correlating in situ lesions and their relationship to nuclear grade enhancement characteristics at MR imaging and mammographic findings are rare. Jansen and colleagues performed a qualitative and quantitative evaluation of 82 consecutively diagnosed pure DCIS lesions on DCE-MRI and classified results according to nuclear grade and x-ray mammographic presentation. In this study, the lesion population was segmented into 4 groups according to their mammographic presentation:
- 1.
Fine pleomorphic, fine linear, or fine linear-branching calcifications (32 of 63)
- 2.
Amorphous or indistinct calcifications (14 of 63)
- 3.
Noncalcified masses (8 of 63)
- 4.
Mammographically occult cases (9 of 63).
Significant differences in the kinetic behavior of these populations were found.
Lesions with fine pleomorphic, fine linear, or fine linear-branching calcifications, and especially those presenting as masses on x-ray mammography, were more suspicious by conventional kinetic standards on MR imaging than lesions with amorphous or indistinct calcifications. In particular, lesions appearing as masses on mammography exhibited washout curves on average, whereas calcifications and occult lesions exhibited plateau curves. Nonmass, clumped enhancement in a segmental or linear distribution predominated. Pure DCIS lesions demonstrated 28% persistent, 27% plateau, and 45% washout behavior, which is similar to distributions found in other reports. For pure DCIS lesions, the average enhancement at 1 minute (E 1 ) was 201%. The reported time to peak enhancement (T peak ) for invasive and benign lesions was 230 seconds, implying that pure DCIS lesions enhance less than invasive cancers and more than benign lesions.
It is interesting that the percent enhancement parameters (E 1 and E peak ) were statistically equivalent across all groups by mammographic and nuclear grade stratification. In this report, the signal enhancement ratio (SER) values were statistically equivalent for pure DCIS lesions of various grades; however, SER values varied by mammographic presentation. This finding suggests that washout as measured by SER may be a more important diagnostic parameter for assessment of in situ lesions than percent enhancement. No significant difference in enhancement kinetics among different nuclear grades of pure DCIS was found.
The findings in this article support previous reports by Viehweg and colleagues, and suggest that x-ray mammographic appearance of pure DCIS may be related to its underlying physiology and vasculature in a way that nuclear grading is not. Additional reports have indicated that perfusion rates increase from benign to DCIS to invasive cancers and are correlated with microvessel density in DCIS lesions. Guidi and colleagues showed an increase in vessel density around ducts with DCIS, although with variable patterns. Heffelfinger and colleagues found that the expression of angiogenic growth factors such as vascular endothelial growth factor increases from hyperplasia to DCIS. Esserman and colleagues studied the relationship between SER values of invasive tumors and tumor vascularity and histologic grade, and found that higher SER values correlate with higher vascularity and higher Scarff-Bloom-Richardson (SBR) pathologic grade.
MR imaging technique
The MR imaging protocols used in the figures shown in this article were performed using 2 1.5-Tesla imaging units (Signa: GE Healthcare, Milwaukee, WI; and Intera Achieva: Philips, the Netherlands). All patients underwent MR imaging in the prone position using parallel imaging technique. A dedicated 8-channel breast coil was used for GE Signa scanner, and a dedicated 7-channel breast coil was used for Philips Intera Achieva scanner. After obtaining bilateral nonfat-saturated T2-weighted images of the breasts, a T1-weighted 3D gradient echo sequence was performed before and 20 seconds after the injection of the contrast material. See Table 1 at the end of this article for imaging protocols. A dynamic study in the axial plane was performed 6 times after the initiation of the intravenous injection of 0.1 mmol/kg gadodiamide (Omniscan; GE Health care) at a rate of 2 mL/s. MR imaging examinations were processed by CADstream (Confirma), and subtraction images and angiogenesis maps were obtained. The images were transferred to a workstation (Advantage Windows, software version 4.0; GE Healthcare, Milwaukee, WI, USA) for analysis.
| Bilateral Nonfat-saturated T2-weighted images | ||||||
| TR/TE | ||||||
| Signa | 5000 ms/103.5 ms | |||||
| Intera Achieva | 16,907 ms/120 ms | |||||
| T1-weighted 3D gradient echo sequence | ||||||
| TR/TE | Flip Angle | Field of View | Matrix | Section Thickness | Acquisition Time | |
| Signa | 4.6 ms / 2.2 ms | 10° | 34 x 34 cm | 320 x 320 | 2 mm | 75 seconds |
| Intera Achieva | 7.9 ms / 3.9 ms | 10° | 48 x 48 cm | 352 x 352 | 2 mm | 75 seconds |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree