Entrapment neuropathies of the hip (ENH) can occur due to a variety of causes with clinical symptoms that may mimic musculoskeletal disorders. Etiologies include entrapment in a fibromuscular canal, tethering due to posttraumatic fibrosis and extrinsic compression from muscle hypertrophy or a mass. Magnetic resonance (MR) imaging enables detection and characterization of peripheral nerve pathology. In addition, MR imaging can impact both diagnostic judgment as well as therapeutic management (nonoperative and operative management) of patients with ENH. This review article will summarize the role of MR imaging in detection, characterization, and management of nerve entrapments around the hip joint.
Key points
- •
Etiologies of entrapment neuropathy around the hip can be variable, including compression in a fibromuscular canal, mass effect due to muscle hypertrophy, and posttraumatic fibrosis.
- •
MR imaging plays a key role in guiding diagnostic thinking and therapeutic management for patients with entrapment neuropathy around the hip joint.
- •
Treatment of entrapment neuropathy can be nonoperative or operative, depending on the severity and etiology.
Introduction
Entrapment neuropathies of the hip (ENH) can be caused by a wide variety of etiologies including trauma that results in traction or compression and regional anatomic factors such as regional muscle hypertrophy, compression or, postsurgical fibrosis. Additionally, ENH may also occur dynamically when extreme ranges of motion are reached during joint movements. , The cumulative prevalence of nerve hip entrapment syndromes has not been discussed in the literature; however, distinct variations of ENH, namely piriformis syndrome (PS), deep gluteal syndrome, and meralgia paresthetica (MP), have a reported prevalence of 0.3% to 6% (among low back pain patients), 0.9% (among patients after hip arthroscopy), and 4.3:10,000 (in the general population). Patients commonly present with pain, sensory alterations, muscle weakness, or a combination of these symptoms depending on the involved nerve. In some patients, the clinical picture can be vague and difficult to localize due to substantial overlap with alternative pathologies, making the diagnosis of entrapment challenging and emphasizing the need for accurate imaging.
Due to its superior soft tissue contrast and ability to visualize detailed anatomy, magnetic resonance (MR) imaging has emerged as a critical tool for diagnosing entrapment neuropathies, providing adjunct data to traditional methods such as electrophysiology. In addition to pinpointing the precise location of nerve entrapment, MR imaging evaluates the surrounding anatomy and can often detect the cause of entrapment, which may guide effective management. Furthermore, MR imaging optimized for the assessment of peripheral nerves, namely MR neurography (MRN), which has also greatly improved in recent years, allows for high-resolution evaluation of the peripheral nerves. , MR imaging plays a key role in determining treatment strategy (nonoperative and operative approaches) and aids in preoperative planning for surgical candidates. MR imaging is also helpful for subsequent assessment of responses to treatment. This review article summarizes the role of MR imaging in the detection and management of various nerve entrapments around the hip.
Anatomy and pathophysiology
The neural structures supplying the hip joint, surrounding pelvic structures, and lower extremities collectively form the lumbosacral plexus (LSP). The LSP consists of the ventral and dorsal rami of spinal nerve roots T12 to S5. Common ENH can be classified based on their origin: lumbar plexus (lateral femoral cutaneous nerve [LFCN], obturator nerve, and femoral nerve [FN]) and sacral plexus (sciatic nerve [SN], posterior femoral cutaneous nerve [PFCN], pudendal nerve [PN], and superior/inferior gluteal nerves).
The pathophysiology of entrapment neuropathies varies. Common mechanisms include compression of the nerve in fibromuscular canal or by ligamentous or muscular structures. Entrapment can occur following surgery, after trauma, or due to chronic myofascial changes that develop with fibrosis or deviations in regional tissues. , Anatomic variants and muscle hypertrophy have also been proposed as mechanisms for entrapment. With chronic compression or traction, microscopic changes occur, characterized by ischemia, edema, intraneural and perineural fibrosis, demyelination, and axonal degeneration; some of these features can be visualized using different MR techniques. Table 1 summarizes the clinical and radiologic features of common ENH.
| Plexus | Nerve | Motor Innervation | Sensory Innervation | Common Etiologies/Risk Factors | Clinical Presentation |
|---|---|---|---|---|---|
| Lumbar | Femoral (ventral L2–L4) | Quadriceps Pectineus Sartorius | Upper and anterior thigh, hip and knee joints | Iliacus or iliopsoas hematoma/fasciitis Femoral artery aneurysms Vascular procedure/access | Diminished knee-jerk reflex. Impaired knee extension (quadriceps femoris) and hip flexion (iliopsoas) Anteromedial thigh and knee, and medial leg paresthesias |
| Obturator (ventral L2–L4 rami) | Adductor magnus Adductor brevis Adductor longus obturator externus Gracilis | Medial and distal thigh | Adductor fascial entrapment Osteitis pubis Chronic adductor tendinopathy Pelvic surgeries | Medial thigh pain | |
| Lateral femoral cutaneous (dorsal L2 and L3) | — | Anterior and lateral thigh | Pregnancy Tight clothing/belt Obesity Hip arthroscopy | Anterolateral thigh pain and paresthesia | |
| Sacral | Sciatic (ventral L4–S3) | Biceps femoris Semitendinosus Semimembranosus Adductor magnus | Gluteal region, posterior thigh, perineum, hip joint, popliteal fossa, and lower leg (except the medial part) | Hip surgery Hip dislocation or fracture Myositis ossificans; intramuscular hemorrhage Posterior paralabral cysts of the hip Compression by piriformis muscle | Sensory, motor, or mixed symptoms depending on the site of entrapment Foot drop |
| Pudendal (ventral rami of S2–S4) | Sphincters of urinary bladder and rectum | External genitalia in both men and women | Entrapment at the ischial spine Entrapment in the pudendal canal between the sacrotuberous and sacrospinous ligaments Long-term cycling | Chronic perineal sensory symptoms | |
| Posterior femoral cutaneous | — | Buttock Perineum Posterior thigh and leg | Hamstring tendon avulsion | Pain, numbness, and paresthesia in inferolateral buttock, perineum, and posterior thigh | |
| Superior gluteal (dorsal rami of L4–S1) | Gluteus medius Gluteus minimus Tensor fasciae latae | — | Hip surgeries Osteophyte formation near the sciatic foramen Intramuscular injection | Weakness in hip abduction | |
| Inferior gluteal (dorsal rami of L5–S2) | Gluteus maximus | — | Hip surgeries Osteophyte formation near the sciatic foramen Sciatic hernia | Weakness in leg extension Gluteus maximus atrophy |
MR Imaging appearance
MRN provides useful features for the diagnosis of nerve entrapment syndromes around the hip joint. The MR characteristics of peripheral nerve disorders include primary alterations in the peripheral nerve itself and secondary skeletal muscle denervation changes from which a nerve abnormality can be deduced. The primary MR features are well seen in large nerves such as the SN, but not easily identified with smaller nerves such as the PCFN or distal PN. Primary nerve abnormalities can include changes in nerve caliber (nerve enlargement), changes in signal intensity (hyperintensity and heterogeneity), intrinsic architectural alterations (disruption of typical neural fascicular structure), deviations in course, perineural findings (blurring of the perifascicular fat or fibrosis), or extrinsic compression (by muscle hypertrophy, fibrosis, or masses such as hematoma or cyst formation from trauma or surgery). , On fat-sensitive sequences (intermediate-weighted, T1-weighted), a normal peripheral nerve exhibits similar to low signal intensity relative to the adjacent skeletal muscle, while on fluid-sensitive sequences (fat-suppressed T2-weighted, short tau inversion recovery [STIR]), it appears with slightly higher signal intensity compared with the adjacent muscle. Therefore, robust fat-sensitive sequences as well as T2-weighted sequences with fat suppression (and potentially vessel suppression as well) are optimal for the detection of peripheral nerve pathology. Although an elevated T2 signal of a peripheral nerve is a sensitive imaging feature of peripheral neuropathy, an alteration in caliber (particularly at a known entrapment site) can add specificity to the diagnosis of entrapment.
The assessment of the regional musculature can aid in identifying the injured nerve. It has been shown that, as early as 4 days, the muscle signal intensity increases on fluid-sensitive sequences after denervation. This increase is proportional to the severity of denervating axonal damage. Therefore, MR imaging can show signal changes in the denervated muscle substantially earlier than the 2 to 3 week period that is needed for electromyographic studies to detect such denervation changes in muscle. , Over time, fatty atrophy of the denervated muscular tissue occurs, and in the chronic setting, this is readily demonstrated on fat-sensitive sequences. ,
Treatment and management
The treatment of entrapment neuropathies ranges from conservative, nonoperative management to operative intervention. Nonoperative treatment options include manual therapy, aerobic conditioning, stretching, strengthening maneuvers that can be performed by patients or assisted by physiotherapists, local anesthetic injections or cryoablation, and cognitive behavioral therapy. , Operative approaches include neurolysis, a procedure in which the nerve is gently freed from surrounding tissues and may be accompanied by the removal of a nerve compressing mass or the drainage of an abscess or hematoma. If conservative treatment and neurolysis are unable to alleviate the patient’s pain, neurectomy and surgical removal of the symptomatic nerve are the last options.
Case studies and imaging examples
Sciatic Neuropathy
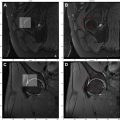
The SN is the largest nerve in the body and is responsible for the motor and sensory innervation of most compartments of the lower limbs. Sciatic neuropathy is the pathologic impairment of the SN before its division into its 2 main branches, the peroneal and tibial nerves, and it encompasses a variety of etiologies. Symptoms of sciatic neuropathy can include upper thigh or lower gluteal pain, radiating pain to the leg, paresthesia below the knee, and foot drop. While nerve damage during total hip replacement surgery is considered the most common etiology (occurring in up to 3.7% of the surgeries), tumoral, ischemic, traumatic, compressive, and idiopathic pathologies have also been named as culprits. , , In the setting of prior hip arthroplasty, a traction injury, changes to alignment, or perineural scarring can result in sciatic neuropathy, while other etiologies can additionally manifest with masses that compress or deviate the nerve. In the context of metal in the field of view (FOV), MR imaging can be optimized with high diagnostic quality for the assessment of LSP and pelvic peripheral nerves. Another specific and underrecognized cause of perineural fibrosis and resultant sciatic neuropathy is related to chronic hamstring avulsion ( Fig. 1 ) injury, present in almost 28% of patients, often requiring mobilization from adherent fibrosis and extensive neurolysis.

Owing to its large size, abnormalities of the SN can be easily visualized on the MR imaging. While SN hyperintensity on fluid-sensitive imaging is a feature of neuropathy, additional characteristics have been specifically described that correlate with the severity of nerve injury and include bulbous enlargement and perineural fibrosis related to traumatic etiology, which in turn can be a cause of entrapment. The treatment of sciatic neuropathy differs based on the etiology and severity of the abnormality and includes medical and surgical management, with primary MR features helping to guide surgical planning. In addition, downstream muscle denervation changes in the leg may be visualized but will require dedicated imaging of the thigh and calf.
Piriformis Syndrome
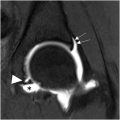
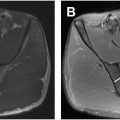
PS, a controversial ENH, is postulated as a specific SN entrapment in the infrapiriformis sciatic foramen between the piriformis muscle (PM) and the upper border of the ischial spine as the nerve crosses the greater sciatic notch or gemelli-obturator complex at the level of ischial tuberosity. , Individuals typically report pain in the gluteal region, often described as a shooting or burning sensation that worsens with sitting, rotation of the hip in flexion, or knee extension; the pain may radiate down the leg. , The “wallet sign,” the inability of a male patient to sit on his wallet without symptoms, has been associated with PS. Hypertrophy of the PM ( Fig. 2 ) can potentially lead to PS, and the PFCN can also be impacted by a hypertrophied PM secondary to compression of the inferior gluteal vein. The occurrence of PS is relatively rare compared to other causes of low back pain and sciatica, with an estimated incidence of 2.4 million cases each year. Although the PS is mostly considered a diagnosis of exclusion, clinical diagnostic criteria have also been proposed for this syndrome. ,

MR imaging may not directly confirm PS but can highlight the presence of anatomic variations in the relevant muscles and nerves that include a split SN, an aberrant nerve route, and a split or hypertrophied PM, potentially related to PS. Traumatic sequelae such as muscle strains and inflammation or nerve-compressing lesions can also be sought as causes of potential PS. , However, while small single-center studies had previously implicated SN variant anatomy as a potential cause of PS, larger retrospective data (1039 consecutive noncontrast adult hip MR examinations) reported the prevalence of variant SN anatomy in 19.2% of patients with no correlation to PS. Furthermore, there is controversy about whether piriformis hypertrophy definitively contributes to nerve compression symptoms and whether addressing the hypertrophy through injections or resection could provide relief. Image-guided interventions have been described to aid the treatment of PS, with injections that aim to reduce muscle inflammation or targeted therapy with botulinum toxin. , , Overall, PS remains a clinical diagnosis, with the primary role of MR imaging being to exclude other causes of sciatic neuropathy and visualize SN and PM anatomy that guide potential injections or contemplated surgical interventions, typically a piriformis tenotomy. , Finally, over time, although the PM can undergo hypertrophy, it can also undergo atrophy in the chronic setting, and asymmetry in the PM bulk should be reported by MR imaging.
Deep Gluteal Syndrome
Accounting for 6% to 8% of sciatic pain, deep gluteal syndrome (DGS) is another specific form of SN compression in the subgluteal space (SGS). Although there is no consensus on the exact definition of the SGS, the most commonly employed definition utilizes the posterior acetabular column, gluteus maximus muscle, gluteal tuberosity, and sacrotuberous ligament as the anterior, posterior, lateral, and medial limits of the SGS, respectively. Furthermore, the sciatic notch and ischial tuberosity define the superior and inferior borders, respectively.
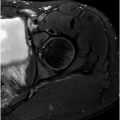
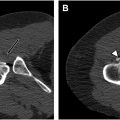
Various etiologies have been reported for DGS, including fibrous bands, PS, trauma, vascular lesions ( Fig. 3 ), anatomic variations, iatrogenic causes, and mechanical overuse. MR imaging and MRN can aid in the diagnosis of DGS by ruling out any spinal or intrapelvic injuries and by visualizing the SGS. SN hyperintensity alone on fluid-sensitive sequences (T2-weighted images) has been shown to be associated with DGS. , Other potential manifestations of DGS include any cause of disruption to the SGS, such as muscle hypertrophy or injury or a mass ( Fig. 4 ) that must be distinguished from the extensive list of pain generators that cause posterior pelvic and buttock region pain.